Highly Diastereo- and Enantioselective Synthesis of Nitrile-Substituted Cyclopropanes by Myoglobin-Mediated Carbene Transfer Catalysis
Dr. Ajay L. Chandgude
Department of Chemistry, University of Rochester, 120 Trustee Road, Rochester, NY, 14627 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Rudi Fasan
Department of Chemistry, University of Rochester, 120 Trustee Road, Rochester, NY, 14627 USA
Search for more papers by this authorDr. Ajay L. Chandgude
Department of Chemistry, University of Rochester, 120 Trustee Road, Rochester, NY, 14627 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Rudi Fasan
Department of Chemistry, University of Rochester, 120 Trustee Road, Rochester, NY, 14627 USA
Search for more papers by this authorGraphical Abstract
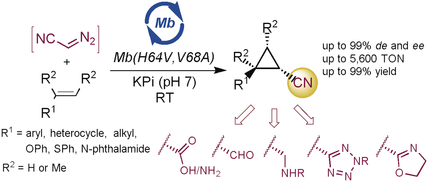
One for many: A chemobiocatalytic strategy involving myoglobin-catalyzed olefin cyclopropanation in the presence of ex situ generated diazoacetonitrile enables the efficient synthesis of a broad range of nitrile-substituted cyclopropanes with high diastereo- and enantioselectivity. The enzymatic product could be further elaborated to afford a variety of functionalized chiral cyclopropanes.
Abstract
A chemobiocatalytic strategy for the highly stereoselective synthesis of nitrile-substituted cyclopropanes is reported. The present approach relies on an asymmetric olefin cyclopropanation reaction catalyzed by an engineered myoglobin in the presence of ex situ generated diazoacetonitrile within a compartmentalized reaction system. This method enabled the efficient transformation of a broad range of olefin substrates at a preparative scale with up to 99.9 % de and ee and up to 5600 turnovers. The enzymatic product could be further elaborated to afford a variety of functionalized chiral cyclopropanes. This work expands the range of synthetically valuable, abiotic transformations accessible through biocatalysis and paves the way to the practical and safe exploitation of diazoacetonitrile in biocatalytic carbene transfer reactions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201810059-sup-0001-misc_information.pdf4.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. P. Doyle, D. C. Forbes, Chem. Rev. 1998, 98, 911–936;
- 1bH. Lebel, J. F. Marcoux, C. Molinaro, A. B. Charette, Chem. Rev. 2003, 103, 977–1050;
- 1cH. M. L. Davies, S. J. Hedley, Chem. Soc. Rev. 2007, 36, 1109–1119;
- 1dH. Pellissier, Tetrahedron 2008, 64, 7041–7095.
- 2T. T. Talele, J. Med. Chem. 2016, 59, 8712–8756.
- 3
- 3aA. J. Fatiadi, Preparation and Synthetic Applications of Cyano Compounds, Wiley-VCH, New York, 1983;
- 3bF. C. Schaefer, The Chemistry of the Cyano Group: Nitrile Reactivity, Wiley Interscience, New York, 1970.
- 4
- 4aJ. Y. Gauthier, N. Chauret, W. Cromlish, S. Desmarais, L. T. Duong, J. P. Falgueyret, D. B. Kimmel, S. Lamontagne, S. Leger, T. LeRiche, C. S. Li, F. Masse, et al., Bioorg. Med. Chem. Lett. 2008, 18, 923–928;
- 4bJ. Pedersen, C. Lauritzen, 2012.
- 5
- 5aJ. R. Denton, K. Cheng, H. M. L. Davies, Chem. Commun. 2008, 1238–1240;
- 5bW. Lin, A. B. Charette, Adv. Synth. Catal. 2005, 347, 1547–1552;
- 5cD. Marcoux, S. Azzi, A. B. Charette, J. Am. Chem. Soc. 2009, 131, 6970–6972;
- 5dS. F. Zhu, X. Xu, J. A. Perman, X. P. Zhang, J. Am. Chem. Soc. 2010, 132, 12796–12799.
- 6Y. Ferrand, P. Le Maux, G. Simonneaux, Tetrahedron: Asymmetry 2005, 16, 3829–3836.
- 7
- 7aR. P. Wurz, A. B. Charette, Org. Lett. 2002, 4, 4531–4533;
- 7bB. Morandi, E. M. Carreira, Angew. Chem. Int. Ed. 2010, 49, 938–941; Angew. Chem. 2010, 122, 950–953;
- 7cB. Morandi, B. Mariampillai, E. M. Carreira, Angew. Chem. Int. Ed. 2011, 50, 1101–1104; Angew. Chem. 2011, 123, 1133–1136.
- 8K. J. Hock, R. Spitzner, R. M. Koenigs, Green Chem. 2017, 19, 2118–2122.
- 9
- 9aP. S. Coelho, E. M. Brustad, A. Kannan, F. H. Arnold, Science 2013, 339, 307–310;
- 9bP. S. Coelho, Z. J. Wang, M. E. Ener, S. A. Baril, A. Kannan, F. H. Arnold, E. M. Brustad, Nat. Chem. Biol. 2013, 9, 485–487;
- 9cM. Bordeaux, V. Tyagi, R. Fasan, Angew. Chem. Int. Ed. 2015, 54, 1744–1748; Angew. Chem. 2015, 127, 1764–1768;
- 9dP. Bajaj, G. Sreenilayam, V. Tyagi, R. Fasan, Angew. Chem. Int. Ed. 2016, 55, 16110–16114; Angew. Chem. 2016, 128, 16344–16348;
- 9eJ. G. Gober, A. E. Rydeen, E. J. Gibson-O'Grady, J. B. Leuthaeuser, J. S. Fetrow, E. M. Brustad, ChemBioChem 2016, 17, 394–397;
- 9fJ. G. Gober, S. V. Ghodge, J. W. Bogart, W. J. Wever, R. R. Watkins, E. M. Brustad, A. A. Bowers, ACS Chem. Biol. 2017, 12, 1726–1731;
- 9gA. Tinoco, V. Steck, V. Tyagi, R. Fasan, J. Am. Chem. Soc. 2017, 139, 5293–5296;
- 9hA. M. Knight, S. B. J. Kan, R. D. Lewis, O. F. Brandenberg, K. Chen, F. H. Arnold, ACS Cent. Sci. 2018, 4, 372–377;
- 9iO. F. Brandenberg, C. K. Prier, K. Chen, A. M. Knight, Z. Wu, F. H. Arnold, ACS Catal. 2018, 8, 2629–2634.
- 10
- 10aP. Srivastava, H. Yang, K. Ellis-Guardiola, J. C. Lewis, Nat. Commun. 2015, 6, 7789;
- 10bG. Sreenilayam, E. J. Moore, V. Steck, R. Fasan, Adv. Synth. Catal. 2017, 359, 2076–2089;
- 10cG. Sreenilayam, E. J. Moore, V. Steck, R. Fasan, ACS Catal. 2017, 7, 7629–7633;
- 10dH. M. Key, P. Dydio, D. S. Clark, J. F. Hartwig, Nature 2016, 534, 534–537;
- 10eH. M. Key, P. Dydio, Z. N. Liu, J. Y. E. Rha, A. Nazarenko, V. Seyedkazemi, D. S. Cark, J. F. Hartwig, ACS Cent. Sci. 2017, 3, 302–308;
- 10fM. W. Wolf, D. A. Vargas, N. Lehnert, Inorg. Chem. 2017, 56, 5623–5635;
- 10gK. Oohora, H. Meichin, L. M. Zhao, M. W. Wolf, A. Nakayama, J. Hasegawa, N. Lehnert, T. Hayashi, J. Am. Chem. Soc. 2017, 139, 17265–17268;
- 10hH. Yang, A. M. Swartz, H. J. Park, P. Srivastava, K. Ellis-Guardiola, D. M. Upp, G. Lee, K. Belsare, Y. F. Gu, C. Zhang, R. E. Moellering, J. C. Lewis, Nat. Chem. 2018, 10, 318–324;
- 10iL. Villarino, K. E. Splan, E. Reddem, L. Alonso-Cotchico, C. Gutiérrez de Souza, A. Lledós, J.-D. Maréchal, A.-M. W. H. Thunnissen, G. Roelfes, Angew. Chem. Int. Ed. 2018, 57, 7785–7789; Angew. Chem. 2018, 130, 7911–7915.
- 11P. K. Mykhailiuk, Eur. J. Org. Chem. 2015, 7235–7239.
- 12
- 12aD. D. Phillips, W. C. Champion, J. Am. Chem. Soc. 1956, 78, 5452;
- 12bM. J. S. Dewar, R. Pettit, J. Chem. Soc. 1956, 2026–2029.
- 13K. J. Hock, L. Mertens, R. Hommelsheim, R. Spitzner, R. M. Koenigs, Chem. Commun. 2017, 53, 6577–6580.
- 14B. J. Anding, A. Ellern, L. K. Woo, Organometallics 2012, 31, 3628–3635.
- 15E. W. Reynolds, T. D. Schwochert, M. W. McHenry, J. W. Watters, E. M. Brustad, ChemBioChem 2017, 18, 2380–2384.
- 16J. Catt, G. Johnson, D. Keavy, R. Mattson, M. Parker, K. Takaki, J. Yevich, U.S. Pat. 5,981,571 Bristol Myers Squibb, 1999.
- 17R. Mago, R. Mahajan, M. E. Thase, Expert Rev. Clin. Pharmacol. 2014, 7, 137–145.
- 18Z. P. Demko, K. B. Sharpless, J. Org. Chem. 2001, 66, 7945–7950.
- 19K. Qiao, X. Yuan, L. Wan, M. W. Zheng, D. Zhang, B. B. Fan, Z. C. Di, Z. Fang, K. Guo, Green Chem. 2017, 19, 5789–5793.
- 20Y. Wei, A. Tinoco, V. Steck, R. Fasan, Y. Zhang, J. Am. Chem. Soc. 2018, 140, 1649–1662.
- 21
- 21aU. T. Bornscheuer, G. W. Huisman, R. J. Kazlauskas, S. Lutz, J. C. Moore, K. Robins, Nature 2012, 485, 185–194;
- 21bM. Hönig, P. Sondermann, N. J. Turner, E. M. Carreira, Angew. Chem. Int. Ed. 2017, 56, 8942–8973; Angew. Chem. 2017, 129, 9068–9100;
- 21cM. T. Reetz, J. Am. Chem. Soc. 2013, 135, 12480–12496;
- 21dM. Hönig, P. Sondermann, N. J. Turner, E. M. Carreira, Angew. Chem. Int. Ed. 2017, 56, 8942–8973; Angew. Chem. 2017, 129, 9068–9100.
- 22CCDC 1872015 (3 a), 1872017 (3c), 1872014 (3d), and 1872016 (3i) contain the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.