Construction of Linear and Branched Tetraboranes by 1,1- and 1,2-Diboration of Diborenes
Dr. Tom E. Stennett
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97070 Würzburg, Germany
Institute for Sustainable Chemistry and Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97070 Würzburg, Germany
Search for more papers by this authorDr. Rüdiger Bertermann
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97070 Würzburg, Germany
Institute for Sustainable Chemistry and Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97070 Würzburg, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Holger Braunschweig
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97070 Würzburg, Germany
Institute for Sustainable Chemistry and Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97070 Würzburg, Germany
Search for more papers by this authorDr. Tom E. Stennett
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97070 Würzburg, Germany
Institute for Sustainable Chemistry and Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97070 Würzburg, Germany
Search for more papers by this authorDr. Rüdiger Bertermann
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97070 Würzburg, Germany
Institute for Sustainable Chemistry and Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97070 Würzburg, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Holger Braunschweig
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97070 Würzburg, Germany
Institute for Sustainable Chemistry and Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97070 Würzburg, Germany
Search for more papers by this authorGraphical Abstract
Abstract
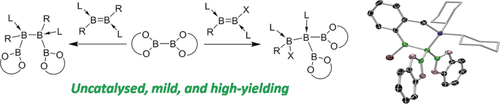
Sterically unencumbered diborenes based on a benzylphosphine chelate undergo diboration reactions with bis(catecholato)diboron in the absence of a catalyst to yield tetraboranes. The symmetrical diborenes studied undergo 1,2-diborations, whereas an unsymmetrical derivative was found to yield a triborylborane–phosphine adduct as the result of a formal 1,1-diboration. A related borylborylene compound also underwent a 1,2-diboration to produce a borylene–borane adduct.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201809976-sup-0001-misc_information.pdf2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Stock, Hydrides of Boron and Silicon, Cornell Univ. Press, Ithaca, New York, 1933;
10.1021/j150356a019 Google Scholar
- 1bW. N. Lipscomb, Adv. Inorg. Chem. 1959, 1, 117–156;
- 1cK. Wade, J. Chem. Soc. Chem. Commun. 1971, 792–793;
- 1dR. E. Williams, Inorg. Chem. 1971, 10, 210–214;
- 1eM. A. Fox, K. Wade, Pure Appl. Chem. 2003, 75, 1315–1323.
- 2E. Osorio, J. K. Olson, W. Tiznado, A. I. Boldyrev, Chem. Eur. J. 2012, 18, 9677–9681.
- 3A. Stock, A. Brandt, H. Fischer, Chem. Ber. 1925, 58, 643–657.
10.1002/cber.19250580402 Google Scholar
- 4
- 4aT. Ishiyama, N. Matsuda, N. Miyaura, A. Suzuki, J. Am. Chem. Soc. 1993, 115, 11018–11019;
- 4bT. Ishiyama, M. Yamamoto, N. Miyaura, Chem. Commun. 1997, 689–690;
- 4cT. Ishiyama, N. Miyaura, Chem. Rec. 2004, 3, 271–280;
- 4dT. B. Marder, N. C. Norman, Top. Catal. 1998, 5, 63–73;
- 4eF. Zhao, X. W. Jia, P. Y. Li, J. W. Zhao, Y. Zhou, J. Wang, H. Liu, Org. Chem. Front. 2017, 4, 2235–2255.
- 5
- 5aA. G. Massey, Adv. Inorg. Chem. 1983, 26, 1–54;
- 5bJ. A. Morrison, Chem. Rev. 1991, 91, 35–48;
- 5cL. Ahmed, J. Castillo, J. A. Morrison, Inorg. Chem. 1992, 31, 1858–1860;
- 5dP. Ceron, A. Finch, J. Frey, J. Kerrigan, T. Parsons, G. Urry, H. I. Schlesinger, J. Am. Chem. Soc. 1959, 81, 6368–6371.
- 6
- 6aE. C. Neeve, S. J. Geier, I. A. I. Mkhalid, S. A. Westcott, T. B. Marder, Chem. Rev. 2016, 116, 9091–9161;
- 6bD. G. Hall, Boronic Acids: Preparation, Applications in Organic Synthesis and Medicine, Wiley-VCH, Weinheim, 2005.
10.1002/3527606548 Google Scholar
- 7
- 7aJ. Huheey, E. Keiter, R. Keiter, Inorganic Chemistry: Principles of Structure and Reactivity, 4th ed., Prentice Hall, Upper Saddle River, 1997;
- 7bH. Braunschweig, R. D. Dewhurst, Angew. Chem. Int. Ed. 2013, 52, 3574–3583; Angew. Chem. 2013, 125, 3658–3667.
- 8
- 8aK. H. Hermannsdörfer, E. Matejčikova, H. Nöth, Chem. Ber.-Recueil 1970, 103, 516–527;
- 8bH. Nöth, H. Pommerening, Angew. Chem. Int. Ed. Engl. 1980, 19, 482–483; Angew. Chem. 1980, 92, 481–482;
- 8cM. Baudler, K. Rockstein, W. Oehlert, Chem. Ber. 1991, 124, 1149–1152;
- 8dM. Arrowsmith, H. Braunschweig, T. E. Stennett, Angew. Chem. Int. Ed. 2017, 56, 96–115; Angew. Chem. 2017, 129, 100–120;
- 8eP. Bissinger, H. Braunschweig, A. Damme, T. Kupfer, A. Vargas, Angew. Chem. Int. Ed. 2012, 51, 9931–9934; Angew. Chem. 2012, 124, 10069–10073;
- 8fR. J. Brotherton, A. L. McCloskey, L. L. Petterson, H. Steinberg, J. Am. Chem. Soc. 1960, 82, 6242–6245;
- 8gH. Braunschweig, R. D. Dewhurst, C. Hörl, A. K. Phukan, F. Pinzner, S. Ullrich, Angew. Chem. Int. Ed. 2014, 53, 3241–3244; Angew. Chem. 2014, 126, 3305–3308;
- 8hT. Kaese, A. Hübner, M. Bolte, H.-W. Lerner, M. Wagner, J. Am. Chem. Soc. 2016, 138, 6224–6233.
- 9
- 9aH. Braunschweig, P. Brenner, R. D. Dewhurst, F. Guethlein, J. O. C. Jiménez-Halla, K. Radacki, J. Wolf, L. Zöllner, Chem. Eur. J. 2012, 18, 8605–8609;
- 9bN. Arnold, H. Braunschweig, R. D. Dewhurst, W. C. Ewing, J. Am. Chem. Soc. 2016, 138, 76–79;
- 9cO. Ciobanu, P. Roquette, S. Leingang, H. Wadepohl, J. Mautz, H. J. Himmel, Eur. J. Inorg. Chem. 2007, 4530–4534.
- 10
- 10aH. Braunschweig, Q. Ye, A. Vargas, R. D. Dewhurst, K. Radacki, A. Damme, Nat. Chem. 2012, 4, 563–567;
- 10bH. Braunschweig, M. Colling, C. H. Hu, K. Radacki, Angew. Chem. Int. Ed. 2002, 41, 1359–1361;
10.1002/1521-3773(20020415)41:8<1359::AID-ANIE1359>3.0.CO;2-J CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 1415–1417.
- 11Y. Hayashi, Y. Segawa, M. Yamashita, K. Nozaki, Chem. Commun. 2011, 47, 5888–5890.
- 12A. Hermann, J. Cid, J. D. Mattock, R. D. Dewhurst, I. Krummenacher, A. Vargas, M. J. Ingleson, H. Braunschweig, Angew. Chem. Int. Ed. 2018, 57, 10091–10095; Angew. Chem. 2018, 130, 10248–10252.
- 13H. Braunschweig, C. Hörl, Chem. Commun. 2014, 50, 10983–10985.
- 14T. E. Stennett, J. D. Mattock, L. Pentecost, A. Vargas, H. Braunschweig, Angew. Chem. Int. Ed. 2018, https://doi.org/10.1002/anie.201809217; Angew. Chem. 2018, https://doi.org/10.1002/ange.201809217.
- 15H. Hommer, H. Nöth, J. Knizek, W. Ponikwar, H. Schwenk-Kircher, Eur. J. Inorg. Chem. 1998, 1519–1527.
10.1002/(SICI)1099-0682(199810)1998:10<1519::AID-EJIC1519>3.0.CO;2-# CAS Web of Science® Google Scholar
- 16L. L. Cao, D. W. Stephan, Organometallics 2017, 36, 3163–3170.
- 17
- 17aP. Nguyen, C. Y. Dai, N. J. Taylor, W. P. Power, T. B. Marder, N. L. Pickett, N. C. Norman, Inorg. Chem. 1995, 34, 4290–4291;
- 17bS. Pietsch, E. C. Neeve, D. C. Apperley, R. Bertermann, F. Y. Mo, D. Qiu, M. S. Cheung, L. Dang, J. B. Wang, U. Radius, Z. Y. Lin, C. Kleeberg, T. B. Marder, Chem. Eur. J. 2015, 21, 7082–7098.
- 18R. D. Dewhurst, E. C. Neeve, H. Braunschweig, T. B. Marder, Chem. Commun. 2015, 51, 9594–9607.
- 19H. Asakawa, K. H. Lee, Z. Y. Lin, M. Yamashita, Nat. Commun. 2014, 5, 4245.
- 20
- 20aM. Gao, S. B. Thorpe, W. L. Santos, Org. Lett. 2009, 11, 3478–3481;
- 20bM. Gao, S. B. Thorpe, C. Kleeberg, C. Slebodnick, T. B. Marder, W. L. Santos, J. Org. Chem. 2011, 76, 3997–4007;
- 20cX. Guo, A. K. Nelson, C. Slebodnick, W. L. Santos, ACS Catal. 2015, 5, 2172–2176;
- 20dA. Bonet, H. Gulyas, E. Fernandez, Angew. Chem. Int. Ed. 2010, 49, 5130–5134; Angew. Chem. 2010, 122, 5256–5260;
- 20eJ. Cid, H. Gulyas, J. J. Carbo, E. Fernandez, Chem. Soc. Rev. 2012, 41, 3558–3570;
- 20fK. S. Lee, A. R. Zhugralin, A. H. Hoveyda, J. Am. Chem. Soc. 2010, 132, 12766.
- 21
- 21aN. Arnold, H. Braunschweig, R. D. Dewhurst, F. Hupp, K. Radacki, A. Trumpp, Chem. Eur. J. 2016, 22, 13927–13934;
- 21bH. Braunschweig, A. Damme, R. D. Dewhurst, T. Kramer, T. Kupfer, K. Radacki, E. Siedler, A. Trumpp, K. Wagner, C. Werner, J. Am. Chem. Soc. 2013, 135, 8702–8707.
- 22T. E. Stennett, J. D. Mattock, I. Vollert, A. Vargas, H. Braunschweig, Angew. Chem. Int. Ed. 2018, 57, 4098–4102; Angew. Chem. 2018, 130, 4162–4167.
- 23H. Asakawa, K. H. Lee, K. Furukawa, Z. Y. Lin, M. Yamashita, Chem. Eur. J. 2015, 21, 4267–4271.
- 24J. Böhnke, H. Braunschweig, A. Deissenberger, T. Dellermann, R. D. Dewhurst, J. O. C. Jimenez-Halla, S. Kachel, H. Kelch, D. Prieschl, Chem. Commun. 2017, 53, 12132–12135.
- 25V. Lillo, M. R. Fructos, J. Ramirez, A. A. C. Braga, F. Maseras, M. M. Díaz-Requejo, P. J. Pérez, E. Fernández, Chem. Eur. J. 2007, 13, 2614–2621.
- 26L. Dang, H. Zhao, Z. Lin, T. B. Marder, Organometallics 2008, 27, 1178–1186.