A Clade II-D Fungal Chimeric Diterpene Synthase from Colletotrichum gloeosporioides Produces Dolasta-1(15),8-diene
Dr. Guangkai Bian
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery, Ministry of Education and School of Pharmaceutical Sciences, Wuhan University, Wuhan, 430071 China
These authors contributed equally to this work.
Search for more papers by this authorJan Rinkel
Kekulé-Institut für Organische Chemie und Biochemie, Rheinische Friedrich-Wilhelms-Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn, Germany
These authors contributed equally to this work.
Search for more papers by this authorDr. Zhangqian Wang
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery, Ministry of Education and School of Pharmaceutical Sciences, Wuhan University, Wuhan, 430071 China
These authors contributed equally to this work.
Search for more papers by this authorLukas Lauterbach
Kekulé-Institut für Organische Chemie und Biochemie, Rheinische Friedrich-Wilhelms-Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn, Germany
Search for more papers by this authorDr. Anwei Hou
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery, Ministry of Education and School of Pharmaceutical Sciences, Wuhan University, Wuhan, 430071 China
Search for more papers by this authorYujie Yuan
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery, Ministry of Education and School of Pharmaceutical Sciences, Wuhan University, Wuhan, 430071 China
Search for more papers by this authorProf. Zixin Deng
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery, Ministry of Education and School of Pharmaceutical Sciences, Wuhan University, Wuhan, 430071 China
Hubei Engineering Laboratory for Synthetic Microbiology, Wuhan Institute of Biotechnology, Wuhan, 430075 China
Search for more papers by this authorCorresponding Author
Prof. Tiangang Liu
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery, Ministry of Education and School of Pharmaceutical Sciences, Wuhan University, Wuhan, 430071 China
Hubei Engineering Laboratory for Synthetic Microbiology, Wuhan Institute of Biotechnology, Wuhan, 430075 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jeroen S. Dickschat
Kekulé-Institut für Organische Chemie und Biochemie, Rheinische Friedrich-Wilhelms-Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn, Germany
Search for more papers by this authorDr. Guangkai Bian
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery, Ministry of Education and School of Pharmaceutical Sciences, Wuhan University, Wuhan, 430071 China
These authors contributed equally to this work.
Search for more papers by this authorJan Rinkel
Kekulé-Institut für Organische Chemie und Biochemie, Rheinische Friedrich-Wilhelms-Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn, Germany
These authors contributed equally to this work.
Search for more papers by this authorDr. Zhangqian Wang
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery, Ministry of Education and School of Pharmaceutical Sciences, Wuhan University, Wuhan, 430071 China
These authors contributed equally to this work.
Search for more papers by this authorLukas Lauterbach
Kekulé-Institut für Organische Chemie und Biochemie, Rheinische Friedrich-Wilhelms-Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn, Germany
Search for more papers by this authorDr. Anwei Hou
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery, Ministry of Education and School of Pharmaceutical Sciences, Wuhan University, Wuhan, 430071 China
Search for more papers by this authorYujie Yuan
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery, Ministry of Education and School of Pharmaceutical Sciences, Wuhan University, Wuhan, 430071 China
Search for more papers by this authorProf. Zixin Deng
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery, Ministry of Education and School of Pharmaceutical Sciences, Wuhan University, Wuhan, 430071 China
Hubei Engineering Laboratory for Synthetic Microbiology, Wuhan Institute of Biotechnology, Wuhan, 430075 China
Search for more papers by this authorCorresponding Author
Prof. Tiangang Liu
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery, Ministry of Education and School of Pharmaceutical Sciences, Wuhan University, Wuhan, 430071 China
Hubei Engineering Laboratory for Synthetic Microbiology, Wuhan Institute of Biotechnology, Wuhan, 430075 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jeroen S. Dickschat
Kekulé-Institut für Organische Chemie und Biochemie, Rheinische Friedrich-Wilhelms-Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn, Germany
Search for more papers by this authorGraphical Abstract
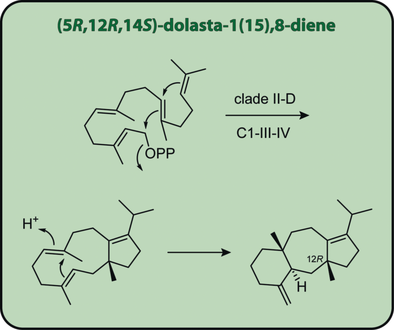
The missing link: Fungal chimeric diterpene synthases with prenyltransferase and terpene synthase domains fall into two clades (I+II) with six subclades (A–F). The present study reports the characterization of the first clade II-D enzyme, the structures of its products, and the cyclization mechanism. The observed 12R configuration reveals a uniform stereochemistry at this carbon for all clade II enzyme products.
Abstract
Based on a terpenoid overproduction platform in yeast for genome mining, a chimeric diterpene synthase from the endophytic fungus Colletotrichum gloeosporioides ES026 was characterized as the (5R,12R,14S)-dolasta-1(15),8-diene synthase. The absolute configuration was independently verified through the use of enantioselectively deuterated terpene precursors, which unequivocally established the predicted C1-III-IV cyclization mode for this first characterized clade II-D enzyme. Extensive isotopic labeling experiments and isolation of the intermediate (1R)-δ-araneosene supported the proposed cyclization mechanism.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201809954-sup-0001-misc_information.pdf3.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. S. Dickschat, Nat. Prod. Rep. 2016, 33, 87;
- 1bY. Yamada, T. Kuzuyama, M. Komatsu, K. Shin-ya, S. Omura, D. E. Cane, H. Ikeda, Proc. Natl. Acad. Sci. USA 2015, 112, 857.
- 2
- 2aT. Mitsuhashi, I. Abe, ChemBioChem 2018, 19, 1106;
- 2bA. Minami, T. Ozaki, C. Liu, H. Oikawa, Nat. Prod. Rep. 2018, https://doi.org/10.1039/C8NP00026C.
- 3G. Appendino, in Progress in the Chemistry of Organic Natural Products (Eds.: ), Springer, Switzerland 2016, pp. 1–90.
- 4F. Berrué, M. W. B. McCulloch, R. G. Kerr, Bioorg. Med. Chem. 2011, 19, 6702.
- 5J. Rinkel, P. Rabe, X. Chen, T. G. Köllner, F. Chen, J. S. Dickschat, Chem. Eur. J. 2017, 23, 10501.
- 6
- 6aM. J. Smanski, Z. Yu, J. Casper, S. Lin, R. M. Peterson, Y. Chen, E. Wendt-Pienkowski, S. R. Rajski, B. Shen, Proc. Natl. Acad. Sci. USA 2011, 108, 13498;
- 6bY.-I. Yang, S. Zhang, K. Ma, Y. Xu, Q. Tao, Y. Chen, J. Chen, S. Guo, J. Ren, W. Wang, Y. Tao, W.-B. Yin, H. Liu, Angew. Chem. Int. Ed. 2017, 56, 4749; Angew. Chem. 2017, 129, 4827.
- 7T. Toyomasu, M. Tsukahara, A. Kaneko, R. Niida, W. Mitsuhashi, T. Dairi, N. Kato, T. Sassa, Proc. Natl. Acad. Sci. USA 2007, 104, 3084.
- 8
- 8aY. Ye, A. Minami, A. Mandi, C. Liu, T. Taniguchi, T. Kuzuyama, K. Monde, K. Gomi, H. Oikawa, J. Am. Chem. Soc. 2015, 137, 11846;
- 8bT. Mitsuhashi, J. Rinkel, M. Okada, I. Abe, J. S. Dickschat, Chem. Eur. J. 2017, 23, 10053.
- 9X.-M. Zhao, Z.-Q. Wang, S.-H. Shu, W.-J. Wang, H.-J. Xu, Y.-J. Ahn, M. Wang, X. Hu, PLoS One 2013, 8, e 61777.
- 10
- 10aC. M. Starks, K. Back, J. Chappell, J. P. Noel, Science 1997, 277, 1815;
- 10bE. Y. Shishova, L. Di Costanzo, D. E. Cane, D. W. Christianson, Biochemistry 2007, 46, 1941.
- 11P. Baer, P. Rabe, K. Fischer, C. A. Citron, T. A. Klapschinski, M. Groll, J. S. Dickschat, Angew. Chem. Int. Ed. 2014, 53, 7652; Angew. Chem. 2014, 126, 7783.
- 12L. C. Tarshis, P. J. Proteau, B. A. Kellogg, J. C. Sacchettini, C. D. Poulter, Proc. Natl. Acad. Sci. USA 1996, 93, 15018.
- 13
- 13aG. Bian, Y. Han, A. Hou, Y. Yuan, X. Liu, Z. Deng, T. Liu, Metab. Eng. 2017, 42, 1;
- 13bG. Bian, A. Hou, Y. Yuan, B. Hu, S. Cheng, Z. Ye, Y. Di, Z. Deng, T. Liu, Org. Lett. 2018, 20, 1626;
- 13cG. Bian, Z. Deng, T. Liu, Curr. Opin. Biotechnol. 2017, 48, 234.
- 14L. Jenny, H.-J. Borschberg, P. Acklin, Tetrahedron 1996, 52, 1549.
- 15P. Rabe, J. Rinkel, E. Dolja, T. Schmitz, B. Nubbemeyer, T. H. Luu, J. S. Dickschat, Angew. Chem. Int. Ed. 2017, 56, 2776; Angew. Chem. 2017, 129, 2820.
- 16H. V. Thulasiram, C. D. Poulter, J. Am. Chem. Soc. 2006, 128, 15819.
- 17L. Lauterbach, J. Rinkel, J. S. Dickschat, Angew. Chem. Int. Ed. 2018, 57, 8280; Angew. Chem. 2018, 130, 8412.
- 18J. W. Cornforth, R. H. Cornforth, G. Popjak, L. Yengoyan, J. Biol. Chem. 1966, 241, 3970.
- 19L. Jenny, H.-J. Borschberg, Helv. Chim. Acta 1995, 78, 715.
- 20
- 20aJ. S. Dickschat, Eur. J. Org. Chem. 2017, 4872;
- 20bT. Toyomasu, M. Tsukahara, H. Kenmoku, M. Anada, H. Nitta, J. Okanda, W. Mitsuhashi, T. Sassa, N. Kato, Org. Lett. 2009, 11, 3044.
- 21
- 21aI. Burkhardt, T. Siemon, M. Henrot, L. Studt, S. Rösler, B. Tudzynski, M. Christmann, J. S. Dickschat, Angew. Chem. Int. Ed. 2016, 55, 8748; Angew. Chem. 2016, 128, 8890;
- 21bJ. Rinkel, L. Lauterbach, P. Rabe, J. S. Dickschat, Angew. Chem. Int. Ed. 2018, 57, 3238; Angew. Chem. 2018, 130, 3292.
- 22P. Rabe, J. Rinkel, T. A. Klapschinski, L. Barra, J. S. Dickschat, Org. Biomol. Chem. 2016, 14, 158.
- 23A. C. Huang, Y. J. Hong, A. D. Bond, D. J. Tantillo, A. Osbourn, Angew. Chem. Int. Ed. 2018, 57, 1291; Angew. Chem. 2018, 130, 1305.
- 24K. A. Rising, C. M. Starks, J. P. Noel, J. Chappell, J. Am. Chem. Soc. 2000, 122, 1861.
- 25G. R. Pettit, R. H. Ode, C. L. Herald, R. B. von Dreel, C. Michel, J. Am. Chem. Soc. 1976, 98, 4677.
- 26M. Ochi, M. Watanabe, I. Miura, M. Taniguchi, T. Tokoroyama, Chem. Lett. 1980, 9, 1229.
- 27C. S. de Figueiredo, S. M. Pinto de Menezes Silva, L. Silva Abreu, E. Ferreira da Silva, M. Sobral da Silva, G. E. Cavalcanti de Miranda, V. C. de O. Costa, M. Le Hyaric, J. Pinto de Sequeira, J. M. B. Filho, J. F. Tavares, Nat. Prod. Res. 2018, https://doi.org/10.1080/14786419.2018.1470512.