Photoredox/Nickel-Catalyzed Single-Electron Tsuji–Trost Reaction: Development and Mechanistic Insights
Dr. Jennifer K. Matsui
Department of Chemistry, University of Pennsylvania, Roy and Diana Vagelos Laboratories, Philadelphia, PA, 19104-6323 USA
Search for more papers by this authorDr. Álvaro Gutiérrez-Bonet
Department of Chemistry, University of Pennsylvania, Roy and Diana Vagelos Laboratories, Philadelphia, PA, 19104-6323 USA
Search for more papers by this authorMadeline Rotella
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD, 20742 USA
Search for more papers by this authorDr. Rauful Alam
Department of Chemistry, University of Pennsylvania, Roy and Diana Vagelos Laboratories, Philadelphia, PA, 19104-6323 USA
Search for more papers by this authorCorresponding Author
Prof. Osvaldo Gutierrez
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD, 20742 USA
Search for more papers by this authorCorresponding Author
Prof. Gary A. Molander
Department of Chemistry, University of Pennsylvania, Roy and Diana Vagelos Laboratories, Philadelphia, PA, 19104-6323 USA
Search for more papers by this authorDr. Jennifer K. Matsui
Department of Chemistry, University of Pennsylvania, Roy and Diana Vagelos Laboratories, Philadelphia, PA, 19104-6323 USA
Search for more papers by this authorDr. Álvaro Gutiérrez-Bonet
Department of Chemistry, University of Pennsylvania, Roy and Diana Vagelos Laboratories, Philadelphia, PA, 19104-6323 USA
Search for more papers by this authorMadeline Rotella
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD, 20742 USA
Search for more papers by this authorDr. Rauful Alam
Department of Chemistry, University of Pennsylvania, Roy and Diana Vagelos Laboratories, Philadelphia, PA, 19104-6323 USA
Search for more papers by this authorCorresponding Author
Prof. Osvaldo Gutierrez
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD, 20742 USA
Search for more papers by this authorCorresponding Author
Prof. Gary A. Molander
Department of Chemistry, University of Pennsylvania, Roy and Diana Vagelos Laboratories, Philadelphia, PA, 19104-6323 USA
Search for more papers by this authorGraphical Abstract
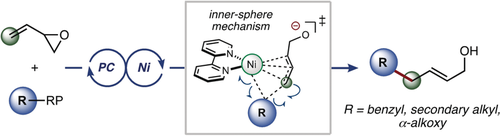
Coming to light: Report herein is a highly regioselective, intermolecular, nickel-catalyzed photoredox allylic substitution that expands both the radical and electrophile scope of dual photoredox/Ni-catalyzed reactions. Quantum mechanical calculations shed light on the mechanistic pathway, supporting a Ni0 to NiII oxidative addition followed by an inner-sphere radical addition. PC=photocatalyst, RP=radical precursor.
Abstract
A regioselective, nickel-catalyzed photoredox allylation of secondary, benzyl, and α-alkoxy radical precursors is disclosed. Through this manifold, a variety of linear allylic alcohols and allylated monosaccharides are accessible in high yields under mild reaction conditions. Quantum mechanical calculations [DFT and DLPNO-CCSD(T)] support the mechanistic hypothesis of a Ni0 to NiII oxidative addition pathway followed by radical addition and inner-sphere allylation.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201809919-sup-0001-misc_information.pdf21.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1B. M. Trost, Acc. Chem. Res. 1980, 13, 385–393.
- 2
- 2aJ. Tsuji, Organic Synthesis with Palladium Compounds, Springer, New York, 1980;
10.1007/978-3-642-67475-4 Google Scholar
- 2bB. M. Trost, D. L. Van Vranken, Chem. Rev. 1996, 96, 395–422.
- 3
- 3aG. Consiglio, R. M. Waymouth, Chem. Rev. 1989, 89, 257–276;
- 3bA. Heumann, M. Reglier, Tetrahedron 1995, 51, 975–1015.
- 4B. M. Trost, M. R. Machacek, A. Aponick, Acc. Chem. Res. 2006, 39, 747.
- 5S.-C. Sha, J. Zhang, P. J. Carroll, P. J. Walsh, J. Am. Chem. Soc. 2013, 135, 17602–17609.
- 6S. Son, G. C. Fu, J. Am. Chem. Soc. 2008, 130, 2756–2757.
- 7
- 7aB. M. Trost, C. R. Self, J. Org. Chem. 1984, 49, 468–473;
- 7bB. M. Trost, E. Keinan, Tetrahedron Lett. 1980, 21, 2591–2594.
- 8E. Negishi, H. Matsushita, S. Chatterjee, R. A. John, J. Org. Chem. 1982, 47, 3188–3190.
- 9M. Braun, T. Meier, Angew. Chem. Int. Ed. 2006, 45, 6952–6955; Angew. Chem. 2006, 118, 7106–7109.
- 10T. Graening, J. F. Hartwig, J. Am. Chem. Soc. 2005, 127, 17192–17193.
- 11B. M. Trost, G. M. Schroeder, J. Am. Chem. Soc. 1999, 121, 6759–6760.
- 12M. Braun, Helv. Chim. Acta 2015, 98, 1–31.
- 13S. B. Lang, K. M. O'Nele, J. A. Tunge, J. Am. Chem. Soc. 2014, 136, 13606–13609.
- 14
- 14aJ. C. Tellis, D. N. Primer, G. A. Molander, Science 2014, 345, 433–436;
- 14bZ. Zuo, D. T. Ahneman, L. Chu, J. A. Terett, A. G. Doyle, D. W. C. MacMillan, Science 2014, 345, 437–440.
- 15
- 15aV. Corcé, L.-M. Chamoreau, E. Derat, J.-P. Goddard, C. Ollivier, L. Fensterbank, Angew. Chem. Int. Ed. 2015, 54, 11414–11418; Angew. Chem. 2015, 127, 11576–11580;
- 15bM. Jouffroy, D. N. Primer, G. A. Molander, J. Am. Chem. Soc. 2016, 138, 475–478;
- 15cK. Nakajima, S. Nojima, Y. Nishibayashi, Angew. Chem. Int. Ed. 2016, 55, 14106–14316; Angew. Chem. 2016, 128, 14312–14316;
- 15dA. Gutiérrez-Bonet, J. C. Tellis, J. K. Matsui, B. A. Vara, G. A. Molander, ACS Catal. 2016, 6, 8004–8008.
- 16Reviews on photoredox catalysis:
- 16aJ. C. Tellis, C. B. Kelly, D. N. Primer, M. Jouffroy, N. R. Patel, G. A. Molander, Acc. Chem. Res. 2016, 49, 1429–1439;
- 16bJ. K. Matsui, S. B. Lang, D. R. Heitz, G. A. Molander, ACS Catal. 2017, 7, 2563–2575;
- 16cC. K. Prier, D. A. Rankic, D. W. C. MacMillan, Chem. Rev. 2013, 113, 5322–5363;
- 16dM. N. Hopkinson, B. Sahoo, J.-L. Li, F. Glorius, Chem. Eur. J. 2014, 20, 3874–3886.
- 17N. R. Patel, C. B. Kelly, M. Jouffroy, G. A. Molander, Org. Lett. 2016, 18, 764–767.
- 18S. Zheng, D. N. Primer, G. A. Molander, ACS Catal. 2017, 7, 7957–7961.
- 19
- 19aJ. Amani, E. Sodagar, G. A. Molander, Org. Lett. 2016, 18, 732–735;
- 19bJ. Amani, G. A. Molander, Org. Lett. 2017, 82, 1856–1863.
- 20J. Amani, G. A. Molander, Org. Lett. 2017, 19, 3612–3615.
- 21J. Amani, R. Alam, S. Badir, G. A. Molander, Org. Lett. 2017, 19, 2426–2429.
- 22S. Z. Tasker, E. A. Standley, T. F. Jamison, Nature 2014, 509, 299–309.
- 23B. M. Trost, G. A. Molander, J. Am. Chem. Soc. 1981, 103, 5969–5972. For an extensive review article, see: J. He, J. Ling, P. Chiu, Chem. Rev. 2014, 114, 8037–8128.
- 24S. Crotti, F. Bertolini, F. Macchia, M. Pineschi, Org. Lett. 2009, 11, 3762. During the course of our studies, Gong and co-workers published a method describing a reductive allylation of tertiary alkyl halides using allylic carbonates: H. Chen, X. Jia, Y. Yu, Q. Qian, H. Gong, Angew. Chem. Int. Ed. 2017, 56, 13103–13106; Angew. Chem. 2017, 129, 13283–13286. Their report includes deuterium studies supporting the formation of a π-allylnickel intermediate.
- 25Secondary alkyltrifluoroborates have significantly high reduction potentials at +1.50 V vs. SCE (see D. N. Primer, K. Karakaya, J. C. Tellis, G. A. Molander, J. Am. Chem. Soc. 2015, 137, 2195–2198).
- 26
- 26aN. Tewari, N. Dwivedi, R. Tripathi, Tetrahedron Lett. 2004, 45, 9011–9014;
- 26bH. Adibi, H. A. Samimi, M. Beygzadeh, Catal. Commun. 2007, 8, 2119–2124;
- 26cA. Heydari, S. Khaksar, M. Tajbakhsh, H. R. Bijanzadeh, J. Fluorine Chem. 2009, 130, 609–614;
- 26dD. Zhang, L.-Z. Wu, L. Zhou, X. Han, Q.-Z. Yang, L.-P. Zhang, C.-H. Tung, J. Am. Chem. Soc. 2004, 126, 3440–3441;
- 26eX. Wei, L. Wang, W. Jia, S. Du, L. Wu, Q. Liu, Chin. J. Chem. 2014, 32, 1245–1250;
- 26fS. Fukuzumi, T. Suenobu, M. Patz, T. Hirasaka, S. Itoh, M. Fujitsuka, O. Ito, J. Am. Chem. Soc. 1998, 120, 8060–8068;
- 26gD. Wang, Q. Liu, B. Chen, L. Zhang, C. Tung, L. Wu, Chin. Sci. Bull. 2010, 55, 2855–2858.
- 27
- 27aK. Nakajima, S. Nojima, K. Sakata, Y. Nishibayashi, ChemCatChem 2016, 8, 1028–1032;
- 27bW. Chen, Z. Liu, J. Tian, J. Li, J. Ma, X. Cheng, G. Li, J. Am. Chem. Soc. 2016, 138, 12312–12315;
- 27cL. Buzzetti, A. Prieto, S. R. Roy, P. Melchiorre, Angew. Chem. Int. Ed. 2017, 56, 15039–15043; Angew. Chem. 2017, 129, 15235–15239;
- 27dC. Verrier, N. Alandini, C. Pezzetta, M. Moliterno, L. Buzzetti, H. B. Hepburn, A. Vega-Penaloza, M. Silvi, P. Melchiorre, ACS Catal. 2018, 8, 1062–1066. For a recent review, see: W. Huang, X. Cheng, Synlett 2017, 28, 148–158.
- 28
- 28aS. O. Badir, A. Dumoulin, J. K. Matsui, G. A. Molander, Angew. Chem. Int. Ed. 2018, 57, 6610–6613; Angew. Chem. 2018, 130, 6720–6723;
- 28bA. Dumoulin, J. K. Matsui, A. Gutierrez-Bonet, G. A. Molander, Angew. Chem. Int. Ed. 2018, 57, 6614–6618; Angew. Chem. 2018, 130, 6724–6728.
- 29N. A. Butt, W. Zhang, Chem. Soc. Rev. 2015, 44, 7929–7967.
- 30
- 30aZ. G. Khalil, A. A. Salim, D. Vuong, A. Crombie, E. Lacey, A. Blumental, R. J. Capon, J. Antibiot. 2017, 70, 1097–1103;
- 30bM. Bayliss, M. I. Donaldson, S. A. Nepogodiev, G. Pergolizzi, A. E. Scott, N. J. Harmer, R. A. Field, J. L. Prior, Carbohydr. Res. 2017, 452, 17–24; synthetic efforts:
- 30cS. Zhao, N. P. Mankad, Angew. Chem. Int. Ed. 2018, 57, 5867–5870; Angew. Chem. 2018, 130, 5969–5972;
- 30dA. Szekrenyi, X. Garrabou, T. Parella, J. Joglar, J. Bujons, P. Clapes, Nat. Chem. 2015, 7, 724–729.
- 31For all of the calculations, dispersion-corrected, broken-spin (U)DFT functionals (UM06/6–311+G(d,p)-SMD(water)//UB3LYP/6-31G(d) and UB3LYP-D3/6–311+G(d,p)-SMD(water)//UB3LYP/6-31G(d)) were used. Further, the energy profile using dynamic correlation, open-shell domain-based local pair natural orbital coupled-cluster calculations (DLPNO-CCSD(T)/def2-TZVPP-SMD(water)//UB3LYP/6-31G(d)) were compared, which are known to provide accurate energies (within 3 kJ mol−1) with the computational cost comparable to DFT calculations. Overall, all methods provided similar conclusions (see the Supporting Information for further details). For simplicity, only UM06/6–311+G(d,p)-SMD(water)//UB3LYP/6-31G(d) energies will be discussed in the manuscript.
- 32O. Gutierrez, J. C. Tellis, D. N. Primer, G. A. Molander, M. C. Kozlowski, J. Am. Chem. Soc. 2015, 137, 4896–4899.
- 33We also considered the protonation of B′′ followed by: 1) outer-sphere C(sp2)−C(sp3) bond formation and 2) radical addition, with subsequent inner-sphere C(sp2)−C(sp3) bond formation. However, the barriers for both protonated inner-sphere and outer-sphere pathways were higher in energy than the anionic pathway (see the Supporting Information).