Selective Degradation of Polo-like Kinase 1 by a Hydrophobically Tagged Inhibitor of the Polo-Box Domain
Stefan Rubner
Leipzig University, Institute of Organic Chemistry, Johannisallee 29, 04103 Leipzig, Germany
These authors contributed equally to this work.
Search for more papers by this authorDr. Andrej Scharow
Leipzig University, Institute of Organic Chemistry, Johannisallee 29, 04103 Leipzig, Germany
These authors contributed equally to this work.
Search for more papers by this authorSabine Schubert
Leipzig University, Institute of Organic Chemistry, Johannisallee 29, 04103 Leipzig, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Thorsten Berg
Leipzig University, Institute of Organic Chemistry, Johannisallee 29, 04103 Leipzig, Germany
Search for more papers by this authorStefan Rubner
Leipzig University, Institute of Organic Chemistry, Johannisallee 29, 04103 Leipzig, Germany
These authors contributed equally to this work.
Search for more papers by this authorDr. Andrej Scharow
Leipzig University, Institute of Organic Chemistry, Johannisallee 29, 04103 Leipzig, Germany
These authors contributed equally to this work.
Search for more papers by this authorSabine Schubert
Leipzig University, Institute of Organic Chemistry, Johannisallee 29, 04103 Leipzig, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Thorsten Berg
Leipzig University, Institute of Organic Chemistry, Johannisallee 29, 04103 Leipzig, Germany
Search for more papers by this authorGraphical Abstract
Abstract
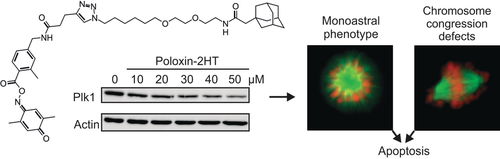
Hydrophobic tagging (HT) of bioactive compounds can induce target degradation via the proteasomal pathway. The first application of hydrophobic tagging to an existing inhibitor of protein–protein interactions is now presented. We developed Poloxin-2HT by fusing an adamantyl tag to Poloxin-2, an inhibitor of the polo-box domain of the protein kinase Plk1, which is a target for tumor therapy. Poloxin-2HT selectively reduced the protein levels of Plk1 in HeLa cells and had a significantly stronger effect on cell viability and the induction of apoptosis than the untagged PBD inhibitor Poloxin-2. The change in cellular phenotype associated with the addition of the hydrophobic tag to Poloxin-2 demonstrated that Poloxin-2HT targets Plk1 in living cells. Our data validate hydrophobic tagging of selective inhibitors of protein–protein interactions as a novel strategy to target and destroy disease-relevant proteins.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201809640-sup-0001-misc_information.pdf962 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1K. Strebhardt, Nat. Rev. Drug Discovery 2010, 9, 643–660.
- 2K. S. Lee, T. R. Burke, Jr., J. E. Park, J. K. Bang, E. Lee, Trends Pharmacol. Sci. 2015, 0, 0.
- 3
- 3aA. E. Elia, L. C. Cantley, M. B. Yaffe, Science 2003, 299, 1228–1231;
- 3bA. E. Elia, P. Rellos, L. F. Haire, J. W. Chao, F. J. Ivins, K. Hoepker, D. Mohammad, L. C. Cantley, S. J. Smerdon, M. B. Yaffe, Cell 2003, 115, 83–95.
- 4
- 4aN. Watanabe, T. Sekine, M. Takagi, J. Iwasaki, N. Imamoto, H. Kawasaki, H. Osada, J. Biol. Chem. 2009, 284, 2344–2353;
- 4bW. Reindl, J. Yuan, A. Krämer, K. Strebhardt, T. Berg, ChemBioChem 2009, 10, 1145–1148;
- 4cY. Chen, J. Zhang, D. Li, J. Jiang, Y. Wang, S. Si, Oncotarget 2017, 8, 1234–1246;
- 4dA. J. Narvaez, S. Ber, A. Crooks, A. Emery, B. Hardwick, E. Guarino Almeida, D. J. Huggins, D. Perera, M. Roberts-Thomson, R. Azzarelli, F. E. Hood, I. A. Prior, D. W. Walker, R. Boyce, R. G. Boyle, S. P. Barker, C. J. Torrance, G. J. McKenzie, A. R. Venkitaraman, Cell Chem. Biol. 2017, 24, 1017–1028;
- 4eA. Berg, T. Berg, ChemBioChem 2016, 17, 650–656.
- 5
- 5aW. Reindl, J. Yuan, A. Krämer, K. Strebhardt, T. Berg, Chem. Biol. 2008, 15, 459–466;
- 5bJ. Yuan, M. Sanhaji, A. Kramer, W. Reindl, M. Hofmann, N. N. Kreis, B. Zimmer, T. Berg, K. Strebhardt, Am. J. Pathol. 2011, 179, 2091–2099.
- 6A. Scharow, M. Raab, K. Saxena, S. Sreeramulu, D. Kudlinzki, S. Gande, C. Dotsch, E. Kurunci-Csacsko, S. Klaeger, B. Kuster, H. Schwalbe, K. Strebhardt, T. Berg, ACS Chem. Biol. 2015, 10, 2570–2579.
- 7
- 7aT. K. Neklesa, H. S. Tae, A. R. Schneekloth, M. J. Stulberg, T. W. Corson, T. B. Sundberg, K. Raina, S. A. Holley, C. M. Crews, Nat. Chem. Biol. 2011, 7, 538–543;
- 7bT. Xie, S. M. Lim, K. D. Westover, M. E. Dodge, D. Ercan, S. B. Ficarro, D. Udayakumar, D. Gurbani, H. S. Tae, S. M. Riddle, T. Sim, J. A. Marto, P. A. Janne, C. M. Crews, N. S. Gray, Nat. Chem. Biol. 2014, 10, 1006–1012;
- 7cJ. L. Gustafson, T. K. Neklesa, C. S. Cox, A. G. Roth, D. L. Buckley, H. S. Tae, T. B. Sundberg, D. B. Stagg, J. Hines, D. P. McDonnell, J. D. Norris, C. M. Crews, Angew. Chem. Int. Ed. 2015, 54, 9659–9662; Angew. Chem. 2015, 127, 9795–9798.
- 8T. K. Neklesa, C. M. Crews, Nature 2012, 487, 308–309.
- 9J. A. Wells, C. L. McClendon, Nature 2007, 450, 1001–1009.
- 10K. Normandin, J. F. Lavallee, M. Futter, A. Beautrait, J. Duchaine, S. Guiral, A. Marinier, V. Archambault, Sci. Rep. 2016, 6, 37581.
- 11Z. Yin, Y. Song, P. H. Rehse, ACS Chem. Biol. 2013, 8, 303–308.
- 12A. Scharow, D. Knappe, W. Reindl, R. Hoffmann, T. Berg, ChemBioChem 2016, 17, 759–767.
- 13
- 13aV. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem. Int. Ed. 2002, 41, 2596–2599;
10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 2708–2711;
- 13bC. W. Tornøe, C. Christensen, M. Meldal, J. Org. Chem. 2002, 67, 3057–3064.
- 14
- 14aW. Reindl, K. Strebhardt, T. Berg, Anal. Biochem. 2008, 383, 205–209;
- 14bW. Reindl, M. Gräber, K. Strebhardt, T. Berg, Anal. Biochem. 2009, 395, 189–194.
- 15K. E. Gascoigne, S. S. Taylor, Cancer Cell 2008, 14, 111–122.
- 16V. Archambault, G. Lepine, D. Kachaner, Oncogene 2015, 34, 4799–4807.
- 17A. Hanisch, A. Wehner, E. A. Nigg, H. H. Sillje, Mol. Biol. Cell 2006, 17, 448–459.
- 18M. Steegmaier, M. Hoffmann, A. Baum, P. Lenart, M. Petronczki, M. Krssak, U. Gurtler, P. Garin-Chesa, S. Lieb, J. Quant, M. Grauert, G. R. Adolf, N. Kraut, J. M. Peters, W. J. Rettig, Curr. Biol. 2007, 17, 316–322.
- 19U. Peters, J. Cherian, J. H. Kim, B. H. Kwok, T. M. Kapoor, Nat. Chem. Biol. 2006, 2, 618–626.
- 20T. U. Mayer, T. M. Kapoor, S. J. Haggarty, R. W. King, S. L. Schreiber, T. J. Mitchison, Science 1999, 286, 971–974.
- 21
- 21aP. M. Cromm, C. M. Crews, Cell Chem. Biol. 2017, 24, 1181–1190;
- 21bA. C. Lai, C. M. Crews, Nat. Rev. Drug Discovery 2017, 16, 101–114.
- 22
- 22aR. Sanchez, J. Meslamani, M. M. Zhou, Biochim. Biophys. Acta Gene Regul. Mech. 2014, 1839, 676–685;
- 22bBromodomains are protein–protein interaction domains that bind to the side chain of acetyl-lysine. Their unusually deep and compact nature makes them atypical protein–protein interaction domains.
- 23
- 23aG. E. Winter, D. L. Buckley, J. Paulk, J. M. Roberts, A. Souza, S. Dhe-Paganon, J. E. Bradner, Science 2015, 348, 1376–1381;
- 23bJ. Lu, Y. Qian, M. Altieri, H. Dong, J. Wang, K. Raina, J. Hines, J. D. Winkler, A. P. Crew, K. Coleman, C. M. Crews, Chem. Biol. 2015, 22, 755–763.
- 24
- 24aK. Raina, J. Lu, Y. Qian, M. Altieri, D. Gordon, A. M. Rossi, J. Wang, X. Chen, H. Dong, K. Siu, J. D. Winkler, A. P. Crew, C. M. Crews, K. G. Coleman, Proc. Natl. Acad. Sci. USA 2016, 113, 7124–7129;
- 24bM. Zengerle, K. H. Chan, A. Ciulli, ACS Chem. Biol. 2015, 10, 1770–1777.
- 25
- 25aD. L. Buckley, J. L. Gustafson, I. Van Molle, A. G. Roth, H. S. Tae, P. C. Gareiss, W. L. Jorgensen, A. Ciulli, C. M. Crews, Angew. Chem. Int. Ed. 2012, 51, 11463–11467; Angew. Chem. 2012, 124, 11630–11634;
- 25bC. Galdeano, M. S. Gadd, P. Soares, S. Scaffidi, I. Van Molle, I. Birced, S. Hewitt, D. M. Dias, A. Ciulli, J. Med. Chem. 2014, 57, 8657–8663.