Observation of Carbodicarbene Ligand Redox Noninnocence in Highly Oxidized Iron Complexes
Dr. Siu-Chung Chan
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore, 637371 Singapore
These authors contributed equally to this work.
Search for more papers by this authorDr. Puneet Gupta
Max-Plank-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
These authors contributed equally to this work.
Search for more papers by this authorXenia Engelmann
Department of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Straße 2, 12489 Berlin, Germany
Search for more papers by this authorZhi Zhong Ang
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore, 637371 Singapore
Search for more papers by this authorDr. Rakesh Ganguly
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore, 637371 Singapore
Search for more papers by this authorDr. Eckhard Bill
Max-Plank-Institut für Chemische Energie Konversion, Stiftstraße 34–36, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorProf. Dr. Kallol Ray
Department of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Straße 2, 12489 Berlin, Germany
Search for more papers by this authorCorresponding Author
Dr. Shengfa Ye
Max-Plank-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorCorresponding Author
Assist. Prof. Jason England
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore, 637371 Singapore
Search for more papers by this authorDr. Siu-Chung Chan
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore, 637371 Singapore
These authors contributed equally to this work.
Search for more papers by this authorDr. Puneet Gupta
Max-Plank-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
These authors contributed equally to this work.
Search for more papers by this authorXenia Engelmann
Department of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Straße 2, 12489 Berlin, Germany
Search for more papers by this authorZhi Zhong Ang
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore, 637371 Singapore
Search for more papers by this authorDr. Rakesh Ganguly
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore, 637371 Singapore
Search for more papers by this authorDr. Eckhard Bill
Max-Plank-Institut für Chemische Energie Konversion, Stiftstraße 34–36, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorProf. Dr. Kallol Ray
Department of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Straße 2, 12489 Berlin, Germany
Search for more papers by this authorCorresponding Author
Dr. Shengfa Ye
Max-Plank-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorCorresponding Author
Assist. Prof. Jason England
Division of Chemistry and Biological Chemistry, School of Physical & Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore, 637371 Singapore
Search for more papers by this authorGraphical Abstract
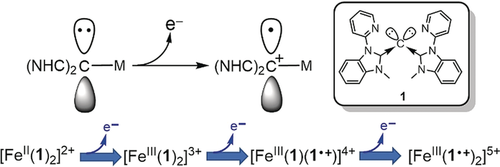
Carbones contain C atoms possessing two lone pairs of electrons. Thus, along with σ-coordination to a metal ion, they have a further pair of electrons, rendering them capable of π-donation or geminal coordination to a second metal. Via formation of a [Fe(1)2]n+ (n=2–5) redox series, where 1 is a tridentate carbodicarbene ligand, it is shown that this second pair of electrons also allows them to be redox-active.
Abstract
To probe the possibility that carbodicarbenes (CDCs) are redox active ligands, all four members of the redox series [Fe(1)2]n+ (n=2–5) were synthesized, where 1 is a neutral tridentate CDC. Through a combination of spectroscopy and DFT calculations, the electronic structure of the pentacation is shown to be [FeIII(1.+)2]5+ (S=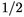 ). That of [Fe(1)2]4+ is more ambiguous, but it has significant contributions from the open-shell singlet [FeIII(1)(1.+)]4+ (S=0). The observed spin states derive from antiferromagnetic coupling of their constituent low-spin iron(III) centres and cation radical ligands. This marks the first time redox activity has been observed for carbones and expands the diverse chemical behaviour known for these ligands.
). That of [Fe(1)2]4+ is more ambiguous, but it has significant contributions from the open-shell singlet [FeIII(1)(1.+)]4+ (S=0). The observed spin states derive from antiferromagnetic coupling of their constituent low-spin iron(III) centres and cation radical ligands. This marks the first time redox activity has been observed for carbones and expands the diverse chemical behaviour known for these ligands.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201809158-sup-0001-misc_information.pdf3.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aF. E. Hahn, M. C. Jahnke, Angew. Chem. Int. Ed. 2008, 47, 3122; Angew. Chem. 2008, 120, 3166;
- 1bM. Melaimi, M. Soleilhavoup, G. Bertrand, Angew. Chem. Int. Ed. 2010, 49, 8810; Angew. Chem. 2010, 122, 8992;
- 1cM. N. Hopkinson, C. Richter, M. Schedler, F. Glorius, Nature 2014, 510, 485.
- 2C. A. Dyker, V. Lavallo, B. Donnadieu, G. Bertrand, Angew. Chem. Int. Ed. 2008, 47, 3206; Angew. Chem. 2008, 120, 3250.
- 3
- 3aA. Fürstner, M. Alcarazo, R. Goddard, C. W. Lehmann, Angew. Chem. Int. Ed. 2008, 47, 3210; Angew. Chem. 2008, 120, 3254;
- 3bM. Alcarazo, C. W. Lehmann, A. Anoop, W. Thiel, A. Fürstner, Nat. Chem. 2009, 1, 295.
- 4F. Ramirez, N. B. Desai, B. Hansen, N. McKelvie, J. Am. Chem. Soc. 1961, 83, 3539.
- 5
- 5aR. Tonner, F. Öxler, B. Neumüller, W. Petz, G. Frenking, Angew. Chem. Int. Ed. 2006, 45, 8038; Angew. Chem. 2006, 118, 8206;
- 5bR. Tonner, G. Frenking, Angew. Chem. Int. Ed. 2007, 46, 8695; Angew. Chem. 2007, 119, 8850;
- 5cR. Tonner, G. Frenking, Chem. Eur. J. 2008, 14, 3260;
- 5dR. Tonner, G. Frenking, Chem. Eur. J. 2008, 14, 3273;
- 5eR. Tonner, G. Heydenrych, G. Frenking, ChemPhysChem 2008, 9, 1474;
- 5fS. Klein, R. Tonner, G. Frenking, Chem. Eur. J. 2010, 16, 10160.
- 6
- 6aH. Schmidbaur, O. Gasser, Angew. Chem. Int. Ed. Engl. 1976, 15, 502; Angew. Chem. 1976, 88, 542;
- 6bJ. Vicente, A. R. Singhal, P. G. Jones, Organometallics 2002, 21, 5887;
- 6cW. Petz, F. Öxler, B. Neumüller, R. Tonner, G. Frenking, Eur. J. Inorg. Chem. 2009, 4507.
- 7
- 7aO. Kaufhold, F. E. Hahn, Angew. Chem. Int. Ed. 2008, 47, 4057; Angew. Chem. 2008, 120, 4122;
- 7bW.-C. Chen, Y.-C. Hsu, C.-Y. Lee, G. P. A. Yap, T.-G. Ong, Organometallics 2013, 32, 2435;
- 7cW.-C. Chen, J.-S. Shen, T. Jurca, C.-J. Peng, Y.-H. Lin, Y.-P. Wang, W.-C. Shih, G. P. A. Yap, T.-G. Ong, Angew. Chem. Int. Ed. 2015, 54, 15207; Angew. Chem. 2015, 127, 15422;
- 7dW.-C. Shih, Y.-T. Chiang, Q. Wang, M.-C. Wu, G. P. A. Yap, L. Zhao, T.-G. Ong, Organometallics 2017, 36, 4287;
- 7eS.-K. Liu, W.-C. Shih, W.-C. Chen, T.-G. Ong, ChemCatChem 2018, 10, 1483.
- 8
- 8aV. Lavallo, C. A. Dyker, B. Donnadieu, G. Bertrand, Angew. Chem. Int. Ed. 2008, 47, 5411; Angew. Chem. 2008, 120, 5491;
- 8bV. Lavallo, C. A. Dyker, B. Donnadieu, G. Bertrand, Angew. Chem. Int. Ed. 2009, 48, 1540; Angew. Chem. 2009, 121, 1568;
- 8cI. Fernández, C. A. Dyker, A. DeHope, B. Donnadieu, G. Frenking, G. Bertrand, J. Am. Chem. Soc. 2009, 131, 11875;
- 8dD. A. Ruiz, M. Melaimi, G. Bertrand, Chem. Asian J. 2013, 8, 2940;
- 8eC. Pranckevicius, L. Liu, G. Bertrand, D. W. Stephan, Angew. Chem. Int. Ed. 2016, 55, 5536; Angew. Chem. 2016, 128, 5626.
- 9
- 9aM. J. Goldfogel, C. C. Roberts, S. J. Meek, J. Am. Chem. Soc. 2014, 136, 6227;
- 9bY.-C. Hsu, J.-S. Shen, B.-C. Lin, W.-C. Chen, Y.-T. Chan, W.-M. Ching, G. P. A. Yap, C.-P. Hsu, T.-G. Ong, Angew. Chem. Int. Ed. 2015, 54, 2420; Angew. Chem. 2015, 127, 2450;
- 9cJ. S. Marcum, C. C. Roberts, R. S. Manan, T. N. Cervarich, S. J. Meek, J. Am. Chem. Soc. 2017, 139, 15580.
- 10
- 10aW.-C. Chen, C.-Y. Lee, B.-C. Lin, Y.-C. Hsu, J.-S. Shen, C.-P. Hsu, G. P. A. Yap, T.-G. Ong, J. Am. Chem. Soc. 2014, 136, 914;
- 10bN. Đorđević, R. Ganguly, M. Petković, D. Vidović, Chem. Commun. 2016, 52, 9789;
- 10cN. Đorđević, R. Ganguly, M. Petković, D. Vidović, Inorg. Chem. 2017, 56, 14671.
- 11
- 11aC. Pranckevicius, L. Fan, D. W. Stephan, J. Am. Chem. Soc. 2015, 137, 5582;
- 11bC. C. Roberts, D. M. Matías, M. J. Goldfogel, S. J. Meek, J. Am. Chem. Soc. 2015, 137, 6488;
- 11cM. J. Goldfogel, S. J. Meek, Chem. Sci. 2016, 7, 4079;
- 11dA. L. Liberman-Martin, R. H. Grubbs, Organometallics 2017, 36, 4091;
- 11eM. J. Goldfogel, C. C. Roberts, R. S. Manan, S. J. Meek, Org. Lett. 2017, 19, 90.
- 12Y.-C. Hsu, V. C.-C. Wang, K.-C. Au-Yeung, C.-Y. Tsai, C.-C. Chang, B.-C. Lin, Y.-T. Chan, C.-P. Hsu, G. P. A. Yap, T. Jurca, T.-G. Ong, Angew. Chem. Int. Ed. 2018, 57, 4622; Angew. Chem. 2018, 130, 4712.
- 13C. Pranckevicius, D. W. Stephan, Organometallics 2013, 32, 2693.
- 14W.-C. Chen, W.-C. Shih, T. Jurca, L. Zhao, D. M. Andrada, C.-J. Peng, C.-C. Chang, S.-K. Liu, Y.-P. Wang, Y.-S. Wen, G. P. A. Yap, C.-P. Hsu, G. Frenking, T.-G. Ong, J. Am. Chem. Soc. 2017, 139, 12830.
- 15
- 15aJ. Sundermeyer, K. Weber, K. Peters, H. G. von Schnering, Organometallics 1994, 13, 2560;
- 15bA. E. Bruce, A. S. Gamble, T. L. Tonker, J. L. Templeton, Organometallics 1987, 6, 1350.
- 16
- 16aP. J. Krusic, U. Klabunde, C. P. Casey, T. F. Block, J. Am. Chem. Soc. 1976, 98, 2015;
- 16bS. Lee, N. J. Cooper, J. Am. Chem. Soc. 1990, 112, 9419.
- 17
- 17aM. A. Sierra, M. G. -Gallego, R. M. -Álvarez, Chem. Eur. J. 2007, 13, 736;
- 17bM. P. Doyle, Angew. Chem. Int. Ed. 2009, 48, 850; Angew. Chem. 2009, 121, 864;
- 17cW. I. Dzik, X. P. Zhang, B. de Bruin, Inorg. Chem. 2011, 50, 9896;
- 17dV. Lyaskovskyy, B. de Bruin, ACS Catal. 2012, 2, 270;
- 17eC. te Grotenhuis, B. de Bruin, Synlett 2018, https://doi.org/10.1055/s-0037-1610204.
- 18
- 18aM. Soleihavoup, G. Bertrand, Acc. Chem. Res. 2015, 48, 256;
- 18bS. Roy, K. C. Mondal, H. W. Roesky, Acc. Chem. Res. 2016, 49, 357;
- 18cK. Chandra Mondal, S. Roy, H. W. Roesky, Chem. Soc. Rev. 2016, 45, 1080;
- 18dM. M. Melaimi, R. Jazzar, M. Soleihavoup, G. Bertrand, Angew. Chem. Int. Ed. 2017, 56, 10046; Angew. Chem. 2017, 129, 10180.
- 19
- 19aK. C. Mondal, H. W. Roesky, M. C. Schwarzer, G. Frenking, I. Tkach, H. Wolf, D. Kratzert, R. Herbst-Irmer, B. Niepötter, D. Stalke, Angew. Chem. Int. Ed. 2013, 52, 1801; Angew. Chem. 2013, 125, 1845;
- 19bP. P. Samuel, K. C. Mondal, H. W. Roesky, M. Hermann, G. Frenking, S. Demeshko, F. Meyer, A. C. Stückl, J. H. Christian, N. S. Dalal, L. Ungur, L. F. Chibotaru, K. Pröpper, A. Meents, B. Dittrich, Angew. Chem. Int. Ed. 2013, 52, 11817; Angew. Chem. 2013, 125, 12033;
- 19cK. C. Mondal, P. P. Samuel, M. Tretiakov, A. P. Singh, H. W. Roesky, A. C. Stückl, B. Niepötter, E. Carl, H. Wolf, R. Herbst-Irmer, D. Stalke, Inorg. Chem. 2013, 52, 4736;
- 19dD. S. Weinberger, M. Melaimi, C. E. Moore, A. L. Rheingold, G. Frenking, P. Jerabek, G. Bertrand, Angew. Chem. Int. Ed. 2013, 52, 8964; Angew. Chem. 2013, 125, 9134;
- 19eA. P. Singh, P. P. Samuel, H. W. Roesky, M. C. Schwarzer, G. Frenking, N. S. Sidhu, B. Dittrich, J. Am. Chem. Soc. 2013, 135, 7324;
- 19fD. S. Weinberger, S. K. Nurul Amin, K. C. Mondal, M. Melaimi, G. Bertrand, A. C. Stückl, H. W. Roesky, B. Dittrich, S. Demeshko, B. Schwederski, W. Kaim, P. Jerabek, G. Frenking, J. Am. Chem. Soc. 2014, 136, 6235;
- 19gM. Arrowsmith, J. Böhnke, H. Braunschweig, M. A. Celik, C. Claes, W. C. Ewing, I. Krummenacher, K. Lubitz, C. Schneider, Angew. Chem. Int. Ed. 2016, 55, 11271; Angew. Chem. 2016, 128, 11441.
- 20
- 20aW. Matheis, W. Kaim, J. Chem. Soc. Faraday Trans. 1990, 86, 3337;
- 20bH. Helten, C. Neumann, A. Espinosa, P. G. Jones, M. Nieger, R. Streubel, Eur. J. Inorg. Chem. 2007, 4669.
- 21CCDC 1857699–1857702 contain the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.