Copper-Catalyzed Ring Opening of Benzofurans and an Enantioselective Hydroamination Cascade
Qing-Feng Xu-Xu
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorQiang-Qiang Liu
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Dr. Xiao Zhang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Shu-Li You
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Collaborative Innovation Center of, Chemical Science and Engineering, Tianjin, 300072 China
Search for more papers by this authorQing-Feng Xu-Xu
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorQiang-Qiang Liu
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Dr. Xiao Zhang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Shu-Li You
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Collaborative Innovation Center of, Chemical Science and Engineering, Tianjin, 300072 China
Search for more papers by this authorGraphical Abstract
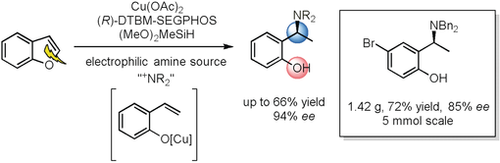
Opening up: A copper(II) acetate/(R)-DTBM-SEGPHOS-catalyzed ring opening of benzofurans and an enantioselective hydroamination cascade with dimethoxymethylsilane (DMMS) and hydroxylamine esters is described. Starting from readily available substituted benzofurans, a series of chiral N,N-dibenzylaminophenols, which are of high interest in pharmaceutical chemistry, were obtained with excellent enantioselectivity.
Abstract
A copper(II) acetate/(R)-DTBM-SEGPHOS-catalyzed ring opening of benzofurans and enantioselective hydroamination cascade with dimethoxymethylsilane (DMMS) and hydroxylamine esters is described. Starting from readily available substituted benzofurans, a series of chiral N,N-dibenzylaminophenols, which are of high interest in pharmaceutical chemistry, were obtained with excellent enantioselectivities (up to 66 % yield, 94 % ee).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201809003-sup-0001-misc_information.pdf6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see:
- 1aA. R. Pape, K. P. Kaliappan, E. P. Kündig, Chem. Rev. 2000, 100, 2917–2940;
- 1bS. Quideau, L. Pouységu, D. Deffieux, Synlett 2008, 467–495;
- 1cL. Pouységu, D. Deffieux, S. Quideau, Tetrahedron 2010, 66, 2235–2261;
- 1dL. Pouységu, T. Sylla, T. Garnier, L. B. Rojas, J. Charris, D. Deffieux, S. Quideau, Tetrahedron 2010, 66, 5908–5917;
- 1eS. P. Roche, J. A. Porco Jr., Angew. Chem. Int. Ed. 2011, 50, 4068–4093; Angew. Chem. 2011, 123, 4154–4179;
- 1fC.-X. Zhuo, W. Zhang, S.-L. You, Angew. Chem. Int. Ed. 2012, 51, 12662–12686; Angew. Chem. 2012, 124, 12834–12858;
- 1gD.-S. Wang, Q.-A. Chen, S.-M. Lu, Y.-G. Zhou, Chem. Rev. 2012, 112, 2557–2590;
- 1hC.-X. Zhuo, C. Zheng, S.-L. You, Acc. Chem. Res. 2014, 47, 2558–2573;
- 1iR. Dalpozzo, Chem. Soc. Rev. 2015, 44, 742–778;
- 1jC. Zheng, S.-L. You, Chem 2016, 1, 830–857;
- 1kW.-T. Wu, L. Zhang, S.-L. You, Chem. Soc. Rev. 2016, 45, 1570–1580;
- 1lW.-T. Wu, L. Zhang, S.-L. You, Acta Chim. Sin. 2017, 75, 419–438;
- 1mJ.-B. Chen, Y.-X. Jia, Org. Biomol. Chem. 2017, 15, 3550–3567; For a book, see:
- 1n Asymmetric Dearomatization Reactions (Ed.: ), Wiley-VCH, Weinheim, 2016.
- 2For selected examples on the asymmetric dearomatization of benzofurans, see:
- 2aM. Maris, W.-R. Huck, T. Mallat, A. Baiker, J. Catal. 2003, 219, 52–58;
- 2bS. Kaiser, S. P. Smidt, A. Pfaltz, Angew. Chem. Int. Ed. 2006, 45, 5194–5197; Angew. Chem. 2006, 118, 5318–5321;
- 2cN. Ortega, S. Urban, B. Beiring, F. Glorius, Angew. Chem. Int. Ed. 2012, 51, 1710–1713; Angew. Chem. 2012, 124, 1742–1745;
- 2dN. Dong, X. Li, F. Wang, J.-P. Cheng, Org. Lett. 2013, 15, 4896–4899;
- 2eJ. Fu, H. Shang, Z. Wang, L. Chang, W. Shao, Z. Yang, Y. Tang, Angew. Chem. Int. Ed. 2013, 52, 4198–4202; Angew. Chem. 2013, 125, 4292–4296;
- 2fT. Shibuta, S. Sato, M. Shibuya, N. Kanoh, T. Taniguchi, K. Monde, Y. Iwabuchi, Heterocycles 2014, 89, 631–639;
- 2gY.-C. Xiao, C.-Z. Yue, P.-Q. Chen, Y.-C. Chen, Org. Lett. 2014, 16, 3208–3211;
- 2hZ. Li, Y. Shi, Org. Lett. 2015, 17, 5752–5755;
- 2iX.-W. Liang, C. Zheng, S.-L. You, Adv. Synth. Catal. 2016, 358, 2066–2071;
- 2jD. Janssen-Müller, M. Fleige, D. Schlüns, M. Wollenburg, C. G. Daniliuc, J. Neugebauer, F. Glorius, ACS Catal. 2016, 6, 5735–5739;
- 2kQ. Tian, J. Bai, B. Chen, G. Zhang, Org. Lett. 2016, 18, 1828–1831;
- 2lQ. Cheng, H.-J. Zhang, W.-J. Yue, S.-L. You, Chem 2017, 3, 428–436;
- 2mB.-X. Xiao, W. Du, Y.-C. Chen, Adv. Synth. Catal. 2017, 359, 1018–1027;
- 2nT.-Z. Li, C.-A. Geng, X.-J. Yin, T.-H. Yang, X.-L. Chen, X.-Y. Huang, Y.-B. Ma, X.-M. Zhang, J.-J. Chen, Org. Lett. 2017, 19, 429–431;
- 2oN. Hu, H. Jung, Y. Zheng, J. Lee, L. Zhang, Z. Ullah, X. Xie, K. Harms, M.-H. Baik, E. Meggers, Angew. Chem. Int. Ed. 2018, 57, 6242–6246; Angew. Chem. 2018, 130, 6350–6354;
- 2pJ.-Q. Zhao, X.-J. Zhou, Y. Zhou, X.-Y. Xu, X.-M. Zhang, W.-C. Yuan, Org. Lett. 2018, 20, 909–912.
- 3For selected examples, see:
- 3aE. Wenkert, E. L. Michelotti, C. S. Swindell, J. Am. Chem. Soc. 1979, 101, 2246–2247;
- 3bH. Kakiya, R. Inoue, H. Shinokubo, K. Oshima, Tetrahedron 2000, 56, 2131–2137;
- 3cJ. Barluenga, L. Álvarez-Rodrigo, F. Rodríguez, F. J. Fañanás, Angew. Chem. Int. Ed. 2004, 43, 3932–3935; Angew. Chem. 2004, 116, 4022–4025;
- 3dJ. Cornella, R. Martin, Org. Lett. 2013, 15, 6298–6301;
- 3eL. Guo, M. Leiendecker, C.-C. Hsiao, C. Baumann, M. Rueping, Chem. Commun. 2015, 51, 1937–1940;
- 3fK. Itami, S. Tanaka, K. Sunahara, G. Tatsuta, A. Mori, Asian J. Org. Chem. 2015, 4, 477–481;
- 3gM. Tobisu, T. Takahira, T. Morioka, N. Chatani, J. Am. Chem. Soc. 2016, 138, 6711–6714;
- 3hT. Iwasaki, R. Akimoto, H. Kuniyasu, N. Kambe, Chem. Asian J. 2016, 11, 2834–2837;
- 3iS. Tsuchiya, H. Saito, K. Nogi, H. Yorimitsu, Org. Lett. 2017, 19, 5557–5560.
- 4For selected examples, see:
- 4aT. Nguyen, E.-i. Negishi, Tetrahedron Lett. 1991, 32, 5903–5906;
- 4bP. Xu, E.-U. Würthwein, C. G. Daniliuc, A. Studer, Angew. Chem. Int. Ed. 2017, 56, 13872–13875; Angew. Chem. 2017, 129, 14060–14063.
- 5For reviews on aromatic metamorphosis, see:
- 5aH. Yorimitsu, D. Vasu, M. Bhanuchandra, K. Murakami, A. Osuka, Synlett 2016, 27, 1765–1774;
- 5bK. Nogi, H. Yorimitsu, Chem. Commun. 2017, 53, 4055–4065; For selected examples, see:
- 5cD. Vasu, H. Yorimitsu, A. Osuka, Angew. Chem. Int. Ed. 2015, 54, 7162–7166; Angew. Chem. 2015, 127, 7268–7272;
- 5dH. Saito, S. Otsuka, K. Nogi, H. Yorimitsu, J. Am. Chem. Soc. 2016, 138, 15315–15318;
- 5eDuring the preparation of this manuscript, the group of Yorimitsu disclosed an elegant copper-catalyzed ring-opening silylation of benzofurans. See: H. Saito, K. Nogi, H. Yorimitsu, Angew. Chem. Int. Ed. 2018, 57, 11030–11034; Angew. Chem. 2018, 130, 11196–11200.
- 6C. K. Hazra, N. Gandhamsetty, S. Park, S. Chang, Nat. Commun. 2016, 7, 13431.
- 7For a selected review, see: E. Vitaku, D. T. Smith, J. T. Njardarson, J. Med. Chem. 2014, 57, 10257–10274.
- 8For reviews on copper hydride-catalyzed hydroamination reactions, see:
- 8aM. T. Pirnot, Y.-M. Wang, S. L. Buchwald, Angew. Chem. Int. Ed. 2016, 55, 48–57; Angew. Chem. 2016, 128, 48–57;
- 8bZ. Sorádová, R. Šebesta, ChemCatChem 2016, 8, 2581–2588.
- 9For selected examples on copper hydride-catalyzed hydroamination reactions, see:
- 9aS. Zhu, N. Niljianskul, S. L. Buchwald, J. Am. Chem. Soc. 2013, 135, 15746–15749;
- 9bY. Miki, K. Hirano, T. Satoh, M. Miura, Angew. Chem. Int. Ed. 2013, 52, 10830–10834; Angew. Chem. 2013, 125, 11030–11034;
- 9cS. Zhu, S. L. Buchwald, J. Am. Chem. Soc. 2014, 136, 15913–15916;
- 9dY. Miki, K. Hirano, T. Satoh, M. Miura, Org. Lett. 2014, 16, 1498–1501;
- 9eN. Niljianskul, S. Zhu, S. L. Buchwald, Angew. Chem. Int. Ed. 2015, 54, 1638–1641; Angew. Chem. 2015, 127, 1658–1661;
- 9fS.-L. Shi, S. L. Buchwald, Nat. Chem. 2015, 7, 38–44;
- 9gD. Niu, S. L. Buchwald, J. Am. Chem. Soc. 2015, 137, 9716–9721;
- 9hD. Nishikawa, K. Hirano, M. Miura, J. Am. Chem. Soc. 2015, 137, 15620–15623;
- 9iY. Yang, S.-L. Shi, D. Niu, P. Liu, S. L. Buchwald, Science 2015, 349, 62–66;
- 9jS. Zhu, N. Niljianskul, S. L. Buchwald, Nat. Chem. 2016, 8, 144–150;
- 9kS.-L. Shi, Z. L. Wong, S. L. Buchwald, Nature 2016, 532, 353–356;
- 9lD. Nishikawa, R. Sakae, Y. Miki, K. Hirano, M. Miura, J. Org. Chem. 2016, 81, 12128–12134;
- 9mY. Xi, T. W. Butcher, J. Zhang, J. F. Hartwig, Angew. Chem. Int. Ed. 2016, 55, 776–780; Angew. Chem. 2016, 128, 786–790;
- 9nH. Wang, J. C. Yang, S. L. Buchwald, J. Am. Chem. Soc. 2017, 139, 8428–8431;
- 9oS. Ichikawa, S. Zhu, S. L. Buchwald, Angew. Chem. Int. Ed. 2018, 57, 8714–8718; Angew. Chem. 2018, 130, 8850–8854;
- 9pT. Takata, D. Nishikawa, K. Hirano, M. Miura, Chem. Eur. J. 2018, 24, 10975–10978;
- 9qR. Y. Liu, S. L. Buchwald, Org. Synth. 2018, 95, 80–96.
- 10For selected examples on mechanistic studies of copper hydride-catalyzed hydroamination, see:
- 10aJ. S. Bandar, M. T. Pirnot, S. L. Buchwald, J. Am. Chem. Soc. 2015, 137, 14812–14818;
- 10bS. Tobisch, Chem. Eur. J. 2016, 22, 8290–8300;
- 10cG. Lu, R. Y. Liu, Y. Yang, C. Fang, D. S. Lambrecht, S. L. Buchwald, P. Liu, J. Am. Chem. Soc. 2017, 139, 16548–16555;
- 10dS. Tobisch, Chem. Sci. 2017, 8, 4410–4423;
- 10eY. Gao, P. Wang, Y. Zhao, Q. Liu, W. Liu, Y. Wang, ChemistrySelect 2018, 3, 2157–2161.
- 11See the Supporting Information for more details.
- 12CCDC 1854512 (3 b) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.