Catalytic Radical Process for Enantioselective Amination of C(sp3)−H Bonds
Dr. Chaoqun Li
Department of Chemistry, University of South Florida, Tampa, FL, 33620 USA
Search for more papers by this authorDr. Kai Lang
Department of Chemistry, Merkert Chemistry Center, Boston College, Chestnut Hill, MA, 02467 USA
Search for more papers by this authorDr. Hongjian Lu
Department of Chemistry, University of South Florida, Tampa, FL, 33620 USA
Search for more papers by this authorDr. Yang Hu
Department of Chemistry, University of South Florida, Tampa, FL, 33620 USA
Search for more papers by this authorDr. Xin Cui
Department of Chemistry, University of South Florida, Tampa, FL, 33620 USA
Search for more papers by this authorDr. Lukasz Wojtas
Department of Chemistry, University of South Florida, Tampa, FL, 33620 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. X. Peter Zhang
Department of Chemistry, Merkert Chemistry Center, Boston College, Chestnut Hill, MA, 02467 USA
Search for more papers by this authorDr. Chaoqun Li
Department of Chemistry, University of South Florida, Tampa, FL, 33620 USA
Search for more papers by this authorDr. Kai Lang
Department of Chemistry, Merkert Chemistry Center, Boston College, Chestnut Hill, MA, 02467 USA
Search for more papers by this authorDr. Hongjian Lu
Department of Chemistry, University of South Florida, Tampa, FL, 33620 USA
Search for more papers by this authorDr. Yang Hu
Department of Chemistry, University of South Florida, Tampa, FL, 33620 USA
Search for more papers by this authorDr. Xin Cui
Department of Chemistry, University of South Florida, Tampa, FL, 33620 USA
Search for more papers by this authorDr. Lukasz Wojtas
Department of Chemistry, University of South Florida, Tampa, FL, 33620 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. X. Peter Zhang
Department of Chemistry, Merkert Chemistry Center, Boston College, Chestnut Hill, MA, 02467 USA
Search for more papers by this authorGraphical Abstract
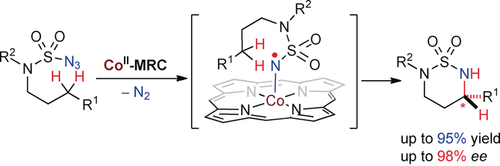
Radicals and Co: An enantioselective radical process has been established using cobalt(II)-based metalloradical catalysis (MRC) for stereoselective amination of aliphatic C−H bonds at room temperature to form chiral 1,3-diamines under neutral and non-oxidative conditions. The cobalt(II)-catalyzed C−H amination features an unusual degree of functional-group tolerance and chemoselectivity.
Abstract
A new catalytic radical system involving CoII-based metalloradical catalysis is effective in activating sulfamoyl azides for enantioselective radical 1,6-amination of C(sp3)−H bonds, affording six-membered chiral heterocyclic sulfamides in high yields with excellent enantioselectivities. The CoII-catalyzed C−H amination features an unusual degree of functional-group tolerance and chemoselectivity. The unique reactivity and stereoselectivity is attributed to the underlying stepwise radical pathway. The resulting optically active cyclic sulfamides can be readily converted into synthetically useful chiral 1,3-diamine derivatives without loss in enantiopurity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201808923-sup-0001-misc_information.pdf7.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. Chatgilialoglu, A. Studer, Encyclopedia of Radicals in Chemistry, Biology and Materials, Wiley, Chichester, 2012;
10.1002/9781119953678 Google Scholar
- 1bD. P. Curran, N. A. Porter, B. Giese, Stereochemistry of Radical Reactions: Concepts, Guidelines, and Synthetic Applications, Wiley-VCH, Weinheim, 2008.
- 2
- 2aM. P. Sibi, S. Manyem, J. Zimmerman, Chem. Rev. 2003, 103, 3263;
- 2bN. Kern, M. P. Plesniak, J. J. W. McDouall, D. J. Procter, Nat. Chem. 2017, 9, 1198.
- 3
- 3aJ. S. Lin, X. Y. Dong, T. T. Li, N. C. Jiang, B. Tan, X. Y. Liu, J. Am. Chem. Soc. 2016, 138, 9357;
- 3bW. Zhang, F. Wang, S. D. McCann, D. Wang, P. Chen, S. S. Stahl, G. Liu, Science 2016, 353, 1014;
- 3cJ. S. Lin, F. L. Wang, X. Y. Dong, W. W. He, Y. Yuan, S. Chen, X. Y. Liu, Nat. Commun. 2017, 8, 14841;
- 3dC. Morrill, C. Jensen, X. Just-Baringo, G. Grogan, N. J. Turner, D. J. Procter, Angew. Chem. Int. Ed. 2018, 57, 3692; Angew. Chem. 2018, 130, 3754.
- 4
- 4aH. Miyabe, A. Kawashima, E. Yoshioka, S. Kohtani, Chem. Eur. J. 2017, 23, 6225;
- 4bA. Studer, D. P. Curran, Angew. Chem. Int. Ed. 2016, 55, 58; Angew. Chem. 2016, 128, 58;
- 4cH. Pellissier, H. Clavier, Chem. Rev. 2014, 114, 2775;
- 4dH. Lu, X. P. Zhang, Chem. Soc. Rev. 2011, 40, 1899;
- 4eT. G. Driver, Org. Biomol. Chem. 2010, 8, 3831;
- 4fM. P. Doyle, Angew. Chem. Int. Ed. 2009, 48, 850; Angew. Chem. 2009, 121, 864.
- 5
- 5aA. Gansäuer, T. Lauterbach, H. Bluhm, M. Noltemeyer, Angew. Chem. Int. Ed. 1999, 38, 2909;
10.1002/(SICI)1521-3773(19991004)38:19<2909::AID-ANIE2909>3.0.CO;2-Y CAS PubMed Web of Science® Google ScholarAngew. Chem. 1999, 111, 3112;10.1002/(SICI)1521-3757(19991004)111:19<3112::AID-ANGE3112>3.0.CO;2-3 Web of Science® Google Scholar
- 5bA. Gansäuer, C.-A. Fan, F. Keller, J. Keil, J. Am. Chem. Soc. 2007, 129, 3484;
- 5cA. Gansäuer, L. Shi, M. Otte, J. Am. Chem. Soc. 2010, 132, 11858;
- 5dS. Hildebrandt, A. Gansäuer, Angew. Chem. Int. Ed. 2016, 55, 9719; Angew. Chem. 2016, 128, 9871;
- 5eN. Funken, F. Mühlhaus, A. Gansäuer, Angew. Chem. Int. Ed. 2016, 55, 12030; Angew. Chem. 2016, 128, 12209;
- 5fW. Hao, X. Wu, J. Z. Sun, J. C. Siu, S. N. MacMillan, S. Lin, J. Am. Chem. Soc. 2017, 139, 12141;
- 5gY.-Q. Zhang, E. Vogelsang, Z.-W. Qu, S. Grimme, A. Gansäuer, Angew. Chem. Int. Ed. 2017, 56, 12654; Angew. Chem. 2017, 129, 12828;
- 5hW. Hao, J. H. Harenberg, X. Wu, S. N. MacMillan, S. Lin, J. Am. Chem. Soc. 2018, 140, 3514.
- 6
- 6aW. I. Dzik, X. Xu, X. P. Zhang, J. N. H. Reek, B. de Bruin, J. Am. Chem. Soc. 2010, 132, 10891;
- 6bJ. L. Belof, C. R. Cioce, X. Xu, X. P. Zhang, B. Space, H. L. Woodcock, Organometallics 2011, 30, 2739;
- 6cH. Lu, W. I. Dzik, X. Xu, L. Wojtas, B. de Bruin, X. P. Zhang, J. Am. Chem. Soc. 2011, 133, 8518.
- 7
- 7aV. Lyaskovskyy, A. I. Olivos Suarez, H. Lu, H. Jiang, X. P. Zhang, B. de Bruin, J. Am. Chem. Soc. 2011, 133, 12264;
- 7bA. I. Olivos Suarez, H. Jiang, X. P. Zhang, B. de Bruin, Dalton Trans. 2011, 40, 5697;
- 7cK. H. Hopmann, A. Ghosh, ACS Catal. 2011, 1, 597;
- 7dM. Goswami, V. Lyaskovskyy, S. R. Domingos, W. J. Buma, S. Woutersen, O. Troeppner, I. Ivanović-Burmazović, H. Lu, X. Cui, X. P. Zhang, E. J. Reijerse, S. DeBeer, M. M. van Schooneveld, F. F. Pfaff, K. Ray, B. de Bruin, J. Am. Chem. Soc. 2015, 137, 5468.
- 8
- 8aY. Chen, K. B. Fields, X. P. Zhang, J. Am. Chem. Soc. 2004, 126, 14718;
- 8bY. Chen, J. V. Ruppel, X. P. Zhang, J. Am. Chem. Soc. 2007, 129, 12074;
- 8cS. Zhu, J. A. Perman, X. P. Zhang, Angew. Chem. Int. Ed. 2008, 47, 8460; Angew. Chem. 2008, 120, 8588;
- 8dS. Fantauzzi, E. Gallo, E. Rose, N. Raoul, A. Caselli, S. Issa, F. Ragaini, S. Cenini, Organometallics 2008, 27, 6143;
- 8eS. Zhu, J. V. Ruppel, H. Lu, L. Wojtas, X. P. Zhang, J. Am. Chem. Soc. 2008, 130, 5042;
- 8fS. Zhu, X. Xu, J. A. Perman, X. P. Zhang, J. Am. Chem. Soc. 2010, 132, 12796;
- 8gX. Xu, H. Lu, J. V. Ruppel, X. Cui, S. Lopez de Mesa, L. Wojtas, X. P. Zhang, J. Am. Chem. Soc. 2011, 133, 15292;
- 8hX. Xu, S. Zhu, X. Cui, L. Wojtas, X. P. Zhang, Angew. Chem. Int. Ed. 2013, 52, 11857; Angew. Chem. 2013, 125, 12073;
- 8iA. R. Reddy, F. Hao, K. Wu, C.-Y. Zhou, C. M. Che, Angew. Chem. Int. Ed. 2016, 55, 1810; Angew. Chem. 2016, 128, 1842;
- 8jY. Wang, X. Wen, X. Cui, L. Wojtas, X. P. Zhang, J. Am. Chem. Soc. 2017, 139, 1049;
- 8kA. Chirila, B. G. Das, N. D. Paul, B. de Bruin, ChemCatChem 2017, 9, 1413;
- 8lX. Xu, Y. Wang, H. X. Cui, L. Wojtas, X. P. Zhang, Chem. Sci. 2017, 8, 4347;
- 8mS. Roy, S. K. Das, B. Chattopadhyay, Angew. Chem. Int. Ed. 2018, 57, 2238; Angew. Chem. 2018, 130, 2260.
- 9
- 9aJ. V. Ruppel, J. E. Jones, C. A. Huff, R. M. Kamble, Y. Chen, X. P. Zhang, Org. Lett. 2008, 10, 1995;
- 9bV. Subbarayan, J. V. Ruppel, S. Zhu, J. A. Perman, X. P. Zhang, Chem. Commun. 2009, 4266;
- 9cL.-M. Jin, X. Xu, H. Lu, X. Cui, L. Wojtas, X. P. Zhang, Angew. Chem. Int. Ed. 2013, 52, 5309; Angew. Chem. 2013, 125, 5417;
- 9dH. Jiang, K. Lang, H. Lu, L. Wojtas, X. P. Zhang, Angew. Chem. Int. Ed. 2016, 55, 11604; Angew. Chem. 2016, 128, 11776;
- 9eH. Jiang, K. Lang, H. Lu, L. Wojtas, X. P. Zhang, J. Am. Chem. Soc. 2017, 139, 9164.
- 10
- 10aX. Cui, X. Xu, L.-M. Jin, L. Wojtas, X. P. Zhang, Chem. Sci. 2015, 6, 1219;
- 10bY. Wang, X. Wen, X. Cui, X. P. Zhang, J. Am. Chem. Soc. 2018, 140, 4792;
- 10cA. S. Karns, M. Goswami, B. de Bruin, Chem. Eur. J. 2018, 24, 5253;
- 10dX. Wen, Y. Wang, X. P. Zhang, Chem. Sci. 2018, 9, 5082.
- 11
- 11aH. Lu, H. Jiang, L. Wojtas, X. P. Zhang, Angew. Chem. Int. Ed. 2010, 49, 10192; Angew. Chem. 2010, 122, 10390;
- 11bH. Lu, V. Subbarayan, J. Tao, X. P. Zhang, Organometallics 2010, 29, 389;
- 11cH. Lu, J. Tao, J. E. Jones, L. Wojtas, X. P. Zhang, Org. Lett. 2010, 12, 1248;
- 11dH. Lu, H. Jiang, Y. Hu, L. Wojtas, X. P. Zhang, Chem. Sci. 2011, 2, 2361;
- 11eH. Lu, Y. Hu, H. Jiang, L. Wojtas, X. P. Zhang, Org. Lett. 2012, 14, 5158;
- 11fL.-M. Jin, H. Lu, Y. Cui, C. L. Lizardi, T. N. Arzua, L. Wojtas, X. Cui, X. P. Zhang, Chem. Sci. 2014, 5, 2422;
- 11gH. Lu, C. Li, H. Jiang, C. L. Lizardi, X. P. Zhang, Angew. Chem. Int. Ed. 2014, 53, 7028; Angew. Chem. 2014, 126, 7148;
- 11hO. Villanueva, N. M. Weldy, S. B. Blakey, C. E. MacBeth, Chem. Sci. 2015, 6, 6672;
- 11iH. Lu, K. Lang, H. Jiang, L. Wojtas, X. P. Zhang, Chem. Sci. 2016, 7, 6934;
- 11jP. F. Kuijpers, M. J. Tiekink, W. B. Breukelaar, D. L. J. Broere, N. P. van Leest, J. I. van der Vlugt, J. N. H. Reek, B. de Bruin, Chem. Eur. J. 2017, 23, 7945;
- 11kZ.-Y. Gu, Y. Liu, F. Wang, X. Bao, S.-Y. Wang, S.-J. Ji, ACS Catal. 2017, 7, 3893.
- 12
- 12aJ.-C. Kizirian, Chem. Rev. 2008, 108, 140;
- 12bX. Ji, H. Huang, Org. Biomol. Chem. 2016, 14, 10557;
- 12cG. Ronquist, R. Hugosson, B. Westermark, J. Cancer Res. Clin. Oncol. 1980, 96, 259.
- 13
- 13aJ. M. Alderson, J. R. Corbin, J. M. Schomaker, Acc. Chem. Res. 2017, 50, 2147;
- 13bF. Collet, C. Lescot, P. Dauban, Chem. Soc. Rev. 2011, 40, 1926;
- 13cY. Liu, X. Guan, E. L.-M. Wong, P. Liu, J.-S. Huang, C.-M. Che, J. Am. Chem. Soc. 2013, 135, 7194;
- 13dJ. M. Alderson, A. M. Phelps, R. J. Scamp, N. S. Dolan, J. M. Schomaker, J. Am. Chem. Soc. 2014, 136, 16720;
- 13eJ.-L. Liang, S.-X. Yuan, J.-S. Huang, W.-Y. Yu, C.-M. Che, Angew. Chem. Int. Ed. 2002, 41, 3465;
10.1002/1521-3773(20020916)41:18<3465::AID-ANIE3465>3.0.CO;2-D CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 3615;
- 13fE. Milczek, N. Boudet, S. Blakey, Angew. Chem. Int. Ed. 2008, 47, 6825; Angew. Chem. 2008, 120, 6931.