Carving Out Pores in Redox-Active One-Dimensional Coordination Polymers
Naomi E. Clayman
Department of Chemistry, Stanford University, Stanford, CA, 94305 USA
These authors contributed equally to this work.
Search for more papers by this authorMary Anne Manumpil
Department of Chemistry, Stanford University, Stanford, CA, 94305 USA
These authors contributed equally to this work.
Search for more papers by this authorDaiki Umeyama
Department of Chemistry, Stanford University, Stanford, CA, 94305 USA
Present Address: International Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, Ibaraki, 305-0044 Japan
Search for more papers by this authorAndrey E. Rudenko
Department of Chemistry, Stanford University, Stanford, CA, 94305 USA
Search for more papers by this authorCorresponding Author
Hemamala I. Karunadasa
Department of Chemistry, Stanford University, Stanford, CA, 94305 USA
Search for more papers by this authorCorresponding Author
Robert M. Waymouth
Department of Chemistry, Stanford University, Stanford, CA, 94305 USA
Search for more papers by this authorNaomi E. Clayman
Department of Chemistry, Stanford University, Stanford, CA, 94305 USA
These authors contributed equally to this work.
Search for more papers by this authorMary Anne Manumpil
Department of Chemistry, Stanford University, Stanford, CA, 94305 USA
These authors contributed equally to this work.
Search for more papers by this authorDaiki Umeyama
Department of Chemistry, Stanford University, Stanford, CA, 94305 USA
Present Address: International Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, Ibaraki, 305-0044 Japan
Search for more papers by this authorAndrey E. Rudenko
Department of Chemistry, Stanford University, Stanford, CA, 94305 USA
Search for more papers by this authorCorresponding Author
Hemamala I. Karunadasa
Department of Chemistry, Stanford University, Stanford, CA, 94305 USA
Search for more papers by this authorCorresponding Author
Robert M. Waymouth
Department of Chemistry, Stanford University, Stanford, CA, 94305 USA
Search for more papers by this authorGraphical Abstract
Abstract
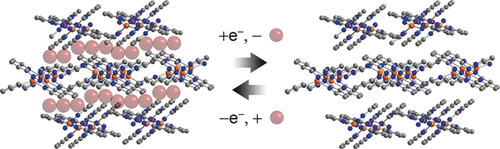
Reduction of the insulating one-dimensional coordination polymer [Cu(abpy)PF6]n, 1 a(PF6), (abpy=2,2′-azobispyridine) yields the conductive, porous polymer [Cu(abpy)]n, 2 a. Pressed pellets of neutral 2 a exhibit a conductivity of 0.093 S cm−1 at room temperature and a Brunauer–Emmett–Teller (BET) surface area of 56 m2 g−1. Fine powders of 2 a have a BET surface area of 90 m2 g−1. Cyclic voltammetry shows that the reduction of 1 a(PF6) to 2 a is quasi-reversible, indicative of facile charge transfer through the bulk material. The BET surface area of the reduced polymer 2 can be controlled by changing the size of the counteranion X in the cationic [Cu(abpy)X]n. Reduction of [Cu(abpy)X]n with X=Br (2 b) or BArF (2 c; BArF=tetrakis(3,5-bis(trifluoromethyl)phenyl)), affords [Cu(abpy)]n polymers with surface areas of 60 and 200 m2 g−1, respectively.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201807506-sup-0001-misc_information.pdf4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. R. Batten, N. R. Champness, X.-M. Chen, J. García-Martínez, S. Kitagawa, L. Öhrström, M. O'Keeffe, M. P. Suh, J. Reedijk, Pure Appl. Chem. 2013, 85, 1715–1724.
- 2G. R. Whittell, M. D. Hager, U. S. Schubert, I. Manners, Nat. Mater. 2011, 10, 176–188.
- 3B. J. Holliday, T. B. Stanford, T. M. Swager, Chem. Mater. 2006, 18, 5649–5651.
- 4B. J. Holliday, T. M. Swager, Chem. Commun. 2005, 23–36.
- 5B. Le Ouay, M. Boudot, T. Kitao, T. Yanagida, S. Kitagawa, T. Uemura, J. Am. Chem. Soc. 2016, 138, 10088–10091.
- 6M. Ballesteros-Rivas, A. Ota, E. Reinheimer, A. Prosvirin, J. Valdés-Martinez, K. R. Dunbar, Angew. Chem. Int. Ed. 2011, 50, 9703–9707; Angew. Chem. 2011, 123, 9877–9881.
- 7Y. Zhang, S. N. Riduan, J. Wang, Chem. Eur. J. 2017, 23, 16419–16431.
- 8N. Casado, G. Hernández, H. Sardon, D. Mecerreyes, Prog. Polym. Sci. 2016, 52, 107–135.
- 9A. G. Slater, A. I. Cooper, Science 2015, 348, 988.
- 10O. Rivada-Wheelaghan, S. L. Aristizábal, J. López-Serrano, R. R. Fayzullin, J. R. Khusnutdinova, Angew. Chem. Int. Ed. 2017, 56, 16267–16271; Angew. Chem. 2017, 129, 16485–16489.
- 11M. Stollenz, J. E. Raymond, L. M. Pérez, J. Wiederkehr, N. Bhuvanesh, Chem. Eur. J. 2016, 22, 2396–2405.
- 12J.-X. Jiang, C. Wang, A. Laybourn, T. Hasell, R. Clowes, Y. Z. Khimyak, J. Xiao, S. J. Higgins, D. J. Adams, A. I. Cooper, Angew. Chem. Int. Ed. 2011, 50, 1072–1075; Angew. Chem. 2011, 123, 1104–1107.
- 13R. P. Kingsborough, T. M. Swager, J. Am. Chem. Soc. 1999, 121, 8825–8834.
- 14P.-L. Vidal, B. Divisia-Blohorn, G. Bidan, J.-L. Hazemann, J.-M. Kern, J.-P. Sauvage, Chem. Eur. J. 2000, 6, 1663–1673.
10.1002/(SICI)1521-3765(20000502)6:9<1663::AID-CHEM1663>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 15W. R. Caseri, H. D. Chanzy, K. Feldman, M. Fontana, P. Smith, T. A. Tervoort, J. G. P. Goossens, E. W. Meijer, A. P. H. J. Schenning, I. P. Dolbnya, M. G. Debije, M. P. de Haas, J. M. Warman, A. M. van de Craats, R. H. Friend, H. Sirringhaus, N. Stutzmann, Adv. Mater. 2003, 15, 125–129.
- 16A. E. Rudenko, N. E. Clayman, J. K. Maclaren, R. M. Waymouth, ChemistrySelect 2016, 1, 3491–3496.
- 17I.-R. Jeon, L. Sun, B. Negru, R. P. Van Duyne, M. Dincă, T. D. Harris, J. Am. Chem. Soc. 2016, 138, 6583–6590.
- 18L. Sun, M. G. Campbell, M. Dincă, Angew. Chem. Int. Ed. 2016, 55, 3566–3579; Angew. Chem. 2016, 128, 3628–3642.
- 19C. S. Campos-Fernández, J. R. Galán-Mascarós, B. W. Smucker, K. R. Dunbar, Eur. J. Inorg. Chem. 2003, 988–994.
- 20S. Maity, S. Kundu, T. Weyhermüller, P. Ghosh, Inorg. Chem. 2015, 54, 1300–1313.
- 21M. B. Robin, P. Day, Adv. Inorg. Chem. Radiochem. 1968, 10, 247–403.
- 22D. M. D'Alessandro, F. R. Keene, Chem. Soc. Rev. 2006, 35, 424–440.
- 23B. Sarkar, S. Patra, J. Fiedler, R. B. Sunoj, D. Janardanan, S. M. Mobin, M. Niemeyer, G. K. Lahiri, W. Kaim, Angew. Chem. Int. Ed. 2005, 44, 5655–5658; Angew. Chem. 2005, 117, 5800–5803.
- 24B. Sarkar, S. Patra, J. Fiedler, R. B. Sunoj, D. Janardanan, G. K. Lahiri, W. Kaim, J. Am. Chem. Soc. 2008, 130, 3532–3542.
- 25J. Takaichi, Y. Morimoto, K. Ohkubo, C. Shimokawa, T. Hojo, S. Mori, H. Asahara, H. Sugimoto, N. Fujieda, N. Nishiwaki, S. Fukuzumi, S. Itoh, Inorg. Chem. 2014, 53, 6159–6169.
- 26K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquérol, T. Siemieniewska, Pure Appl. Chem. 1985, 57, 603–619.
- 27M. Rose, N. Klein, W. Böhlmann, B. Böhringer, S. Fichtner, S. Kaskel, Soft Matter 2010, 6, 3918–3923.
- 28D. K. Schroder, Semiconductor Material and Device Characterization, 3rd ed., Wiley, Hoboken, 2006.
- 29CCDC 1825140 contains the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.