The Mackay-Type Cluster [Cu43Al12](Cp*)12: Open-Shell 67-Electron Superatom with Emerging Metal-Like Electronic Structure
Dr. Jana Weßing
Chair of Inorganic and Metal-Organic Chemistry, Technical University of Munich, Lichtenbergstrasse 4, 85748 Garching, Germany
Search for more papers by this authorDr. Chelladurai Ganesamoorthy
University of Duisburg-Essen, Faculty of Chemistry, Universitätstrasse 5–7, 45141 Essen, Germany
Search for more papers by this authorDr. Samia Kahlal
Univ Rennes, CNRS, ISCR-UMR 6226, 35000 Rennes, France
Search for more papers by this authorDr. Rémi Marchal
Univ Rennes, CNRS, ISCR-UMR 6226, 35000 Rennes, France
Search for more papers by this authorDr. Christian Gemel
Chair of Inorganic and Metal-Organic Chemistry, Technical University of Munich, Lichtenbergstrasse 4, 85748 Garching, Germany
Search for more papers by this authorProf. Dr. Olivier Cador
Univ Rennes, CNRS, ISCR-UMR 6226, 35000 Rennes, France
Search for more papers by this authorDr. Augusto C. H. Da Silva
São Carlos Institute of Chemistry, University of São Paulo, PO Box 780, 13560-970, São Carlos São Paulo, Brazil
Search for more papers by this authorProf.Dr. Juarez L. F. Da Silva
São Carlos Institute of Chemistry, University of São Paulo, PO Box 780, 13560-970, São Carlos São Paulo, Brazil
Search for more papers by this authorCorresponding Author
Prof. Dr. Jean-Yves Saillard
Univ Rennes, CNRS, ISCR-UMR 6226, 35000 Rennes, France
Search for more papers by this authorCorresponding Author
Prof. Dr. Roland A. Fischer
Chair of Inorganic and Metal-Organic Chemistry, Technical University of Munich, Lichtenbergstrasse 4, 85748 Garching, Germany
Search for more papers by this authorDr. Jana Weßing
Chair of Inorganic and Metal-Organic Chemistry, Technical University of Munich, Lichtenbergstrasse 4, 85748 Garching, Germany
Search for more papers by this authorDr. Chelladurai Ganesamoorthy
University of Duisburg-Essen, Faculty of Chemistry, Universitätstrasse 5–7, 45141 Essen, Germany
Search for more papers by this authorDr. Samia Kahlal
Univ Rennes, CNRS, ISCR-UMR 6226, 35000 Rennes, France
Search for more papers by this authorDr. Rémi Marchal
Univ Rennes, CNRS, ISCR-UMR 6226, 35000 Rennes, France
Search for more papers by this authorDr. Christian Gemel
Chair of Inorganic and Metal-Organic Chemistry, Technical University of Munich, Lichtenbergstrasse 4, 85748 Garching, Germany
Search for more papers by this authorProf. Dr. Olivier Cador
Univ Rennes, CNRS, ISCR-UMR 6226, 35000 Rennes, France
Search for more papers by this authorDr. Augusto C. H. Da Silva
São Carlos Institute of Chemistry, University of São Paulo, PO Box 780, 13560-970, São Carlos São Paulo, Brazil
Search for more papers by this authorProf.Dr. Juarez L. F. Da Silva
São Carlos Institute of Chemistry, University of São Paulo, PO Box 780, 13560-970, São Carlos São Paulo, Brazil
Search for more papers by this authorCorresponding Author
Prof. Dr. Jean-Yves Saillard
Univ Rennes, CNRS, ISCR-UMR 6226, 35000 Rennes, France
Search for more papers by this authorCorresponding Author
Prof. Dr. Roland A. Fischer
Chair of Inorganic and Metal-Organic Chemistry, Technical University of Munich, Lichtenbergstrasse 4, 85748 Garching, Germany
Search for more papers by this authorThis work is dedicated to Prof. Dr. Dr. h.c. mult. Wolfgang A. Herrmann on the occasion of his 70th birthday
Graphical Abstract
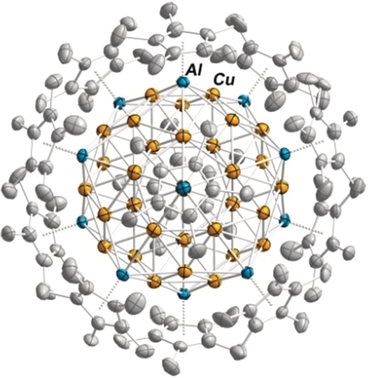
Superatomic: The reaction of [AlCp*]4 and [CuMes]5 yields the Mackay 55-metal atom two-shell icosahedral cluster [Cu43Al12](Cp*)12. The intermetalloid cluster has a unique 67-electron open-shell superatomic [Cu43Al12]12+ core, embedded inside an all-hydrocarbon shell of twelve Cp*− ligands. Its electronic structure comprises a set of entangled HOMO und low-lying LUMOs, prefiguring the formation of a conduction band.
Abstract
The paramagnetic cluster [Cu43Al12](Cp*)12 was obtained from the reaction of [CuMes]5 and [AlCp*]4 (Cp*=η5-C5Me5; Mes=mesityl). This all-hydrocarbon ligand-stabilized M55 magic atom-number cluster features a Mackay-type nested icosahedral structure. Its open-shell 67-electron superatom configuration is unique. Three unpaired electrons occupy weakly antibonding jellium states. The situation prefigures the formation of a conduction band, which is in line with the measured temperature-independent magnetism. Steric protection by twelve Cp* ligands suppresses the intrinsic polyradicalar reactivity of the Cu43Al12 core.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201806039-sup-0001-misc_information.pdf1.7 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. Brack, Rev. Mod. Phys. 1993, 65, 677–732.
- 2Z. Luo, A. W. Castleman, Acc. Chem. Res. 2014, 47, 2931–2940.
- 3M. Walter, J. Akola, O. Lopez-Acevedo, P. D. Jadzinsky, G. Calero, C. J. Ackerson, R. L. Whetten, H. Grönbeck, H. Häkkinen, Proc. Natl. Acad. Sci. USA 2008, 105, 9157–9162.
- 4J. Vollet, J. R. Hartig, H. Schnöckel, Angew. Chem. Int. Ed. 2004, 43, 3186–3189; Angew. Chem. 2004, 116, 3248–3252.
- 5P. A. Clayborne, O. Lopez-Acevedo, R. L. Whetten, H. Grönbeck, H. Häkkinen, Eur. J. Inorg. Chem. 2011, 2649–2652.
- 6M. Huber, A. Schnepf, C. E. Anson, H. Schnöckel, Angew. Chem. Int. Ed. 2008, 47, 8201–8206; Angew. Chem. 2008, 120, 8323–8328.
- 7S. Gonzalez-Gallardo, G. Prabusankar, T. Cadenbach, C. Gemel, M. von Hopffgarten, G. Frenking, R. A. Fischer, In Metal-Metal Bonding (Ed.: ), Springer, Berlin, Heidelberg, 2010, pp. 147–188.
- 8S. González-Gallardo, T. Bollermann, R. A. Fischer, R. Murugavel, Chem. Rev. 2012, 112, 3136–3170.
- 9P. W. Roesky, Dalton Trans. 2009, 1887–1893.
- 10C. Gemel, T. Steinke, M. Cokoja, A. Kempter, R. A. Fischer, Eur. J. Inorg. Chem. 2004, 4161–4176.
- 11G. Linti, H. Schnöckel, Coord. Chem. Rev. 2000, 206–207, 285–319.
- 12J. D. Erickson, E. G. Mednikov, S. A. Ivanov, L. F. Dahl, J. Am. Chem. Soc. 2016, 138, 1502–1505.
- 13A. Mackay, Acta Crystallogr. 1962, 15, 916–918.
- 14M. v. Laue, Z. Kristallogr. 1943, 105, 124.
10.1524/zkri.1943.105.1.124 Google Scholar
- 15M. J. Piotrowski, C. G. Ungureanu, P. Tereshchuk, K. E. A. Batista, A. S. Chaves, D. Guedes-Sobrinho, J. L. F. Da Silva, J. Phys. Chem. C 2016, 120, 28844–28856.
- 16T. Rapps, R. Ahlrichs, E. Waldt, M. M. Kappes, D. Schooss, Angew. Chem. Int. Ed. 2013, 52, 6102–6105; Angew. Chem. 2013, 125, 6218–6221.
- 17N. T. Tran, D. R. Powell, L. F. Dahl, Angew. Chem. Int. Ed. 2000, 39, 4121–4125;
10.1002/1521-3773(20001117)39:22<4121::AID-ANIE4121>3.0.CO;2-A CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 4287–4291.
- 18E. G. Mednikov, M. C. Jewell, L. F. Dahl, J. Am. Chem. Soc. 2007, 129, 11619–11630.
- 19A. Dass, S. Theivendran, P. R. Nimmala, C. Kumara, V. R. Jupally, A. Fortunelli, L. Sementa, G. Barcaro, X. Zuo, B. C. Noll, J. Am. Chem. Soc. 2015, 137, 4610–4613.
- 20G. D. S. Douglas, M. C. Henrique, G. R. Gustavo, F. d. O. Marcelo, L. F. D. S. Juarez, J. Phys. Condens. Matter 2016, 28, 175302.
- 21Y. Grin, F. R. Wagner, M. Armbrüster, M. Kohout, A. Leithe-Jasper, U. Schwarz, U. Wedig, H. Georg von Schnering, J. Solid State Chem. 2006, 179, 1707–1719.
- 22S. Westman, Acta Chem. Scand. 1965, 19, 1411–1419.
- 23L. Arnberg, S. Westman, Acta Crystallogr. Sect. A 1978, 34, 399–404.
- 24T. Steinke, C. Gemel, M. Cokoja, M. Winter, R. A. Fischer, Angew. Chem. Int. Ed. 2004, 43, 2299–2302; Angew. Chem. 2004, 116, 2349–2352.
- 25T. Steinke, M. Cokoja, C. Gemel, A. Kempter, A. Krapp, G. Frenking, U. Zenneck, R. A. Fischer, Angew. Chem. Int. Ed. 2005, 44, 2943–2946; Angew. Chem. 2005, 117, 3003–3007.
- 26T. Steinke, C. Gemel, M. Winter, R. A. Fischer, Chem. Eur. J. 2005, 11, 1636–1646.
- 27C. Ganesamoorthy, J. Weßing, C. Kroll, R. W. Seidel, C. Gemel, R. A. Fischer, Angew. Chem. Int. Ed. 2014, 53, 7943–7947; Angew. Chem. 2014, 126, 8077–8081.
- 28A. Bondi, J. Phys. Chem. 1964, 68, 441–451.
- 29M. Mantina, A. C. Chamberlin, R. Valero, C. J. Cramer, D. G. Truhlar, J. Phys. Chem. A 2009, 113, 5806–5812.
- 30R. W. G. Wyckoff, Crystal Structures, Vol. 1, 2nd ed., Interscience Publishers, New York, 1963.
- 31T.-A. D. Nguyen, Z. R. Jones, B. R. Goldsmith, W. R. Buratto, G. Wu, S. L. Scott, T. W. Hayton, J. Am. Chem. Soc. 2015, 137, 13319–13324.
- 32T.-A. D. Nguyen, Z. R. Jones, D. F. Leto, G. Wu, S. L. Scott, T. W. Hayton, Chem. Mater. 2016, 28, 8385–8390.
- 33D. Bono, J. Hartig, M. Huber, H. Schnöckel, L. J. de Jongh, J. Cluster Sci. 2007, 18, 319–331.
- 34H.-J. Himmel, J. Vollet, Organometallics 2002, 21, 5972–5977.
- 35V. Blum, R. Gehrke, F. Hanke, P. Havu, V. Havu, X. Ren, K. Reuter, M. Scheffler, Comput. Phys. Commun. 2009, 180, 2175–2196.
- 36V. Havu, V. Blum, P. Havu, M. Scheffler, J. Comput. Phys. 2009, 228, 8367–8379.
- 37C. Fonseca Guerra, J. G. Snijders, G. te Velde, E. J. Baerends, Theor. Chem. Acc. 1998, 99, 391–403.
- 38G. te Velde, F. M. Bickelhaupt, E. J. Baerends, C. Fonseca Guerra, S. J. A. van Gisbergen, J. G. Snijders, T. Ziegler, J. Comput. Chem. 2001, 22, 931–967.
- 39SCM, ADF2017, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands.
- 40J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 1996, 77, 3865–3868.
- 41A. D. Becke, J. Chem. Phys. 1986, 84, 4524–4529.
- 42A. D. Becke, Phys. Rev. A 1988, 38, 3098–3100.
- 43J. P. Perdew, Phys. Rev. B 1986, 33, 8822–8824.
- 44J. P. Perdew, Phys. Rev. B 1986, 34, 7406–7406.
- 45O. Kahn, Molecular Magnetism, VCH, Weinheim, 1993.
- 46X.-K. Wan, X.-L. Cheng, Q. Tang, Y.-Z. Han, G. Hu, D.-e. Jiang, Q.-M. Wang, J. Am. Chem. Soc. 2017, 139, 9451–9454.
- 47X. Liu, D. Astruc, Coord. Chem. Rev. 2018, 359, 112–126.
- 48A. W. Cook, Z. R. Jones, G. Wu, S. L. Scott, T. W. Hayton, J. Am. Chem. Soc. 2018, 140, 394–400.
- 49K. K. Chakrahari, J.-H. Liao, S. Kahlal, Y.-C. Liu, M.-H. Chiang, J.-Y. Saillard, C. W. Liu, Angew. Chem. Int. Ed. 2016, 55, 14704–14708; Angew. Chem. 2016, 128, 14924–14928.
- 50From the point of view of a possible cluster formation process, the energy gain obtained by the coordination of 12 AlCp units to Cu43 was computed to be 1641 kJ mol−1 (spin-restricted calculations), a value similar to that found for Al50Cp*12. Ref. [4]
- 51J. Li, X. Li, H.-J. Zhai, L.-S. Wang, Science 2003, 299, 864–867.
- 52K. Freitag, H. Banh, C. Gemel, R. W. Seidel, S. Kahlal, J.-Y. Saillard, R. A. Fischer, Chem. Commun. 2014, 50, 8681–8684.
- 53I. Chakraborty, T. Pradeep, Chem. Rev. 2017, 117, 8208–8271.
- 54R. Jin, C. Zeng, M. Zhou, Y. Chen, Chem. Rev. 2016, 116, 10346–10413.