Transition-Metal-Like Behavior of Monovalent Boron Compounds: Reduction, Migration, and Complete Cleavage of CO at a Boron Center
Dr. Hao Wang
Department of Chemistry and State Key Laboratory of Synthetic Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong, China
Search for more papers by this authorLinlin Wu
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhenyang Lin
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zuowei Xie
Department of Chemistry and State Key Laboratory of Synthetic Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong, China
Search for more papers by this authorDr. Hao Wang
Department of Chemistry and State Key Laboratory of Synthetic Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong, China
Search for more papers by this authorLinlin Wu
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhenyang Lin
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zuowei Xie
Department of Chemistry and State Key Laboratory of Synthetic Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong, China
Search for more papers by this authorGraphical Abstract
Abstract
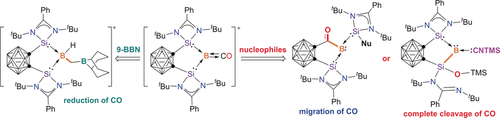
The borylene–carbonyl moiety in [bis(silylene)B(CO)][WBr(CO)5] shows diverse reactivity. Reduction, migration, and complete cleavage of CO have been observed at the boron center, leading to the formation of new types of borylenes. These reactions not only serve as new methods for the synthesis of various stable borylenes, but also demonstrate that main-group-element compounds can mimic the behavior of transition-metal complexes.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201802643-sup-0001-misc_information.pdf1.8 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. P. Power, Nature 2010, 463, 171;
- 1bD. Martin, M. Soleilhavoup, G. Bertrand, Chem. Sci. 2011, 2, 389.
- 2
- 2aD. W. Stephan, G. Erker, Angew. Chem. Int. Ed. 2010, 49, 46; Angew. Chem. 2010, 122, 50;
- 2bD. W. Stephan, G. Erker, Angew. Chem. Int. Ed. 2015, 54, 6400; Angew. Chem. 2015, 127, 6498;
- 2cD. W. Stephan, Acc. Chem. Res. 2015, 48, 306;
- 2dD. W. Stephan, J. Am. Chem. Soc. 2015, 137, 10018;
- 2eD. W. Stephan, Science 2016, 354, aaf 7229.
- 3
- 3aP. P. Power, Acc. Chem. Res. 2011, 44, 627;
- 3bS. Yao, Y. Xiong, M. Driess, Organometallics 2011, 30, 1748;
- 3cS. K. Mandal, H. W. Roesky, Acc. Chem. Res. 2012, 45, 298;
- 3dS. Khan, S. S. Sen, H. W. Roesky, Chem. Commun. 2012, 48, 2169;
- 3eS. Yadav, S. Saha, S. S. Sen, ChemCatChem 2016, 8, 486;
- 3fM. Arrowsmith, H. Braunschweig, T. E. Stennett, Angew. Chem. Int. Ed. 2017, 56, 96; Angew. Chem. 2017, 129, 100;
- 3gM. Melaimi, R. Jazzar, M. Soleilhavoup, G. Bertrand, Angew. Chem. Int. Ed. 2017, 56, 10046; Angew. Chem. 2017, 129, 10180.
- 4
- 4aA. T. Biju, N. Kuhl, F. Glorius, Acc. Chem. Res. 2011, 44, 1182;
- 4bM. N. Hopkinson, C. Richter, M. Schedler, F. Glorius, Nature 2014, 510, 485;
- 4cM. H. Wang, K. A. Scheidt, Angew. Chem. Int. Ed. 2016, 55, 14912; Angew. Chem. 2016, 128, 15134;
- 4dC. Zhang, J. F. Hooper, D. W. Lupton, ACS Catal. 2017, 7, 2583.
- 5G. C. Welch, R. R. S. Juan, J. D. Masuda, D. W. Stephan, Science 2006, 314, 1124.
- 6
- 6aM. Soleilhavoup, G. Bertrand, Angew. Chem. Int. Ed. 2017, 56, 10282; Angew. Chem. 2017, 129, 10416;
- 6bR. Kinjo, B. Donnadieu, M. A. Celik, G. Frenking, G. Bertrand, Science 2011, 333, 610;
- 6cD. A. Ruiz, M. Melaimi, G. Bertrand, Chem. Commun. 2014, 50, 7837;
- 6dL. Kong, Y. Li, R. Ganguly, D. Vidovic, R. Kinjo, Angew. Chem. Int. Ed. 2014, 53, 9280; Angew. Chem. 2014, 126, 9434;
- 6eF. Dahcheh, D. Martin, D. W. Stephan, G. Bertrand, Angew. Chem. Int. Ed. 2014, 53, 13159; Angew. Chem. 2014, 126, 13375;
- 6fA. D. Ledet, T. W. Hudnall, Dalton Trans. 2016, 45, 9820;
- 6gH. Braunschweig, R. D. Dewhurst, F. Hupp, M. Nutz, K. Radacki, C. W. Tate, A. Vargas, Q. Ye, Nature 2015, 522, 327;
- 6hH. Braunschweig, M. A. Celik, R. D. Dewhurst, K. Ferkinghoff, A. Hermann, J. O. C. Jimenez-Halla, T. Kramer, K. Radacki, R. Shang, E. Siedler, F. Weißenberger, C. Werner, Chem. Eur. J. 2016, 22, 11736;
- 6iH. Wang, J. Zhang, Z. Lin, Z. Xie, Chem. Commun. 2015, 51, 16817;
- 6jM. Arrowsmith, D. Auerhammer, R. Bertermann, H. Braunschweig, G. Bringmann, M. A. Celik, R. D. Dewhurst, M. Finze, M. Grüne, M. Hailmann, T. Hertle, I. Krummenacher, Angew. Chem. Int. Ed. 2016, 55, 14464; Angew. Chem. 2016, 128, 14680;
- 6kA. Rosas-Sánchez, I. Alvarado-Beltran, A. Baceiredo, D. Hashizume, N. Saffon-Merceron, V. Branchadell, T. Kato, Angew. Chem. Int. Ed. 2017, 56, 4814; Angew. Chem. 2017, 129, 4892;
- 6lH. Braunschweig, I. Krummenacher, M.-A. Légaré, A. Matler, K. Radacki, Q. Ye, J. Am. Chem. Soc. 2017, 139, 1802;
- 6mM. Arrowsmith, D. Auerhammer, R. Bertermann, H. Braunschweig, M. A. Celik, J. Erdmannsdörfer, I. Krummenacher, T. Kupfer, Angew. Chem. Int. Ed. 2017, 56, 11263; Angew. Chem. 2017, 129, 11417;
- 6nM.-A. Légaré, G. Bélanger-Chabot, R. D. Dewhurst, E. Welz, I. Krummenacher, B. Engels, H. Braunschweig, Science 2018, 359, 896;
- 6oL. Kong, W. Lu, L. Yongxin, R. Ganguly, R. Kinjo, Inorg. Chem. 2017, 56, 5586;
- 6pL. Kong, W. Lu, Y. Li, R. Ganguly, R. Kinjo, Angew. Chem. Int. Ed. 2016, 55, 14718; Angew. Chem. 2016, 128, 14938.
- 7
- 7aY. Wang, G. H. Robinson, Inorg. Chem. 2011, 50, 12326;
- 7bP. Bissinger, H. Braunschweig, A. Damme, R. D. Dewhurst, T. Kupfer, K. Radacki, K. Wagner, J. Am. Chem. Soc. 2011, 133, 19044;
- 7cY.-L. Rao, L. D. Chen, N. J. Mosey, S. Wang, J. Am. Chem. Soc. 2012, 134, 11026.
- 8W. J. Grigsby, P. P. Power, J. Am. Chem. Soc. 1996, 118, 7981.
- 9H. Wang, J. Zhang, H. K. Lee, Z. Xie, J. Am. Chem. Soc. 2018, 140, 3888.
- 10H. Wang, L. Wu, Z. Lin, Z. Xie, J. Am. Chem. Soc. 2017, 139, 13680.
- 11
- 11aM. W. Rathke, H. C. Brown, J. Am. Chem. Soc. 1966, 88, 2606;
- 11bM. Sajid, L.-M. Elmer, C. Rosorius, C. G. Daniliuc, S. Grimme, G. Kehr, G. Erker, Angew. Chem. Int. Ed. 2013, 52, 2243; Angew. Chem. 2013, 125, 2299;
- 11cM. Sajid, G. Kehr, C. G. Daniliuc, G. Erker, Angew. Chem. Int. Ed. 2014, 53, 1118; Angew. Chem. 2014, 126, 1136;
- 11dL. D. Curless, E. R. Clark, J. Cid, A. D. Grosso, M. J. Ingleson, Chem. Commun. 2015, 51, 10903.
- 12
- 12aM. L. Lepage, S. Lai, N. Peressin, R. Hadjerci, B. O. Patrick, D. M. Perrin, Angew. Chem. Int. Ed. 2017, 56, 15257; Angew. Chem. 2017, 129, 15459;
- 12bJ. Taguchi, T. Ikeda, R. Takahashi, I. Sasaki, Y. Ogasawara, T. Dairi, N. Kato, Y. Yamamoto, J. W. Bode, H. Ito, Angew. Chem. Int. Ed. 2017, 56, 13847; Angew. Chem. 2017, 129, 14035.
- 13
- 13aM. Arrowsmith, J. Böhnke, H. Braunschweig, M. A. Celik, Angew. Chem. Int. Ed. 2017, 56, 14287; Angew. Chem. 2017, 129, 14475;
- 13bJ. Böhnke, H. Braunschweig, T. Dellermann, W. C. Ewing, K. Hammond, J. O. C. Jimenez-Halla, T. Kramer, J. Mies, Angew. Chem. Int. Ed. 2015, 54, 13801; Angew. Chem. 2015, 127, 14006;
- 13cH. Braunschweig, T. Dellermann, R. D. Dewhurst, W. C. Ewing, K. Hammond, J. O. C. Jimenez-Halla, T. Kramer, I. Krummenacher, J. Mies, A. K. Phukan, A. Vargas, Nat. Chem. 2013, 5, 1025;
- 13dH. Asakawa, K.-H. Lee, Z. Lin, M. Yamashita, Nat. Commun. 2014, 5, 5245;
- 13eR. Dobrovetsky, D. W. Stephan, J. Am. Chem. Soc. 2013, 135, 4974;
- 13fX. Wang, Z. Zhu, Y. Peng, H. Lei, J. C. Fettinger, P. P. Power, J. Am. Chem. Soc. 2009, 131, 6912.
- 14CCDC 1823722 ([2][WBr(CO)5]), 1823723 (3), 1823724 (4), and 1823725 (5) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.