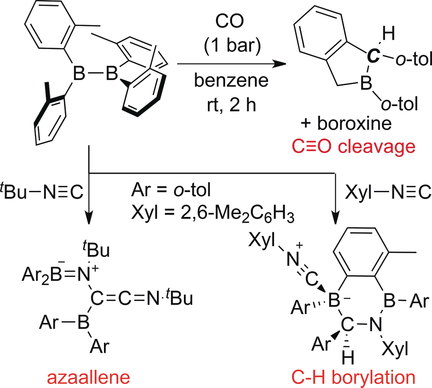
Reaction of B2(o-tol)4 with CO and Isocyanides: Cleavage of the C≡O Triple Bond and Direct C−H Borylations
Yuhei Katsuma
Department of Applied Chemistry, Faculty of Science and Engineering, Chuo University, 1-13-27 Kasuga, Bunkyo-ku, 112-8551 Tokyo, Japan
Search for more papers by this authorNana Tsukahara
Department of Applied Chemistry, Faculty of Science and Engineering, Chuo University, 1-13-27 Kasuga, Bunkyo-ku, 112-8551 Tokyo, Japan
Search for more papers by this authorLinlin Wu
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhenyang Lin
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Makoto Yamashita
Department of Molecular and Macromolecular Chemistry, Graduate School of Engineering, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, 464-8603 Aichi Japan
Search for more papers by this authorYuhei Katsuma
Department of Applied Chemistry, Faculty of Science and Engineering, Chuo University, 1-13-27 Kasuga, Bunkyo-ku, 112-8551 Tokyo, Japan
Search for more papers by this authorNana Tsukahara
Department of Applied Chemistry, Faculty of Science and Engineering, Chuo University, 1-13-27 Kasuga, Bunkyo-ku, 112-8551 Tokyo, Japan
Search for more papers by this authorLinlin Wu
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhenyang Lin
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Makoto Yamashita
Department of Molecular and Macromolecular Chemistry, Graduate School of Engineering, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, 464-8603 Aichi Japan
Search for more papers by this authorGraphical Abstract
Borane and CO: The reaction between highly Lewis acidic (o-tol)2B−B(o-tol)2 and CO afforded a mixture of boraindane and boroxine by complete cleavage of the C≡O triple bond. 13C labeling experiments demonstrated that the carbon atom in boraindane stems from CO. The reaction of diborane(4) with tBu−NC afforded an azaallene, while the reaction with Xyl−NC afforded cyclic compounds by direct C−H borylations.
Abstract
The reaction of highly Lewis acidic tetra(o-tolyl)diborane(4) with CO afforded a mixture of boraindane and boroxine by the cleavage of the C≡O triple bond. 13C labeling experiments confirmed that the carbon atom in the boraindane stems from CO. Simultaneously, formation of boroxine 3 could be considered as borylene transfer to capture the oxygen atom from CO. The reaction of diborane(4) with tBu−NC afforded an azaallene, while the reaction with Xyl−NC furnished cyclic compounds by direct C−H borylations.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201800878-sup-0001-misc_information.pdf1.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. F. Hartwig, in Organotransition Metal Chemistry: From Bonding to Catalysis, University Science Books, Sausalito, CA, 2010.
- 2
- 2aA. B. Burg, H. I. Schlesinger, J. Am. Chem. Soc. 1937, 59, 780–787;
- 2bW. J. J. M. Sprangers, R. Louw, J. Chem. Soc. Perkin Trans. 2 1976, 1895–1901;
- 2cD. Seyferth, R. M. Weinstein, J. Am. Chem. Soc. 1982, 104, 5534–5535.
- 3
- 3aP. Paetzold, B. Redenz-Stormanns, R. Boese, Angew. Chem. Int. Ed. Engl. 1990, 29, 900–902; Angew. Chem. 1990, 102, 910–911;
- 3bS. Luckert, E. Eversheim, M. Müller, B. Redenz-Stormanns, U. Englert, P. Paetzold, Chem. Ber. 1995, 128, 1029–1035;
- 3cJ. Teichmann, H. Stock, H. Pritzkow, W. Siebert, Eur. J. Inorg. Chem. 1998, 459–463;
- 3dM. R. Mason, B. Song, K. Kirschbaum, J. Am. Chem. Soc. 2004, 126, 11812–11813;
- 3eN. Nakata, T. Oikawa, T. Matsumoto, Y. Kabe, A. Sekiguchi, Organometallics 2005, 24, 3368–3370;
- 3fV. Lavallo, Y. Canac, B. Donnadieu, W. W. Schoeller, G. Bertrand, Angew. Chem. Int. Ed. 2006, 45, 3488–3491; Angew. Chem. 2006, 118, 3568–3571;
- 3gX. Li, C. Ni, H. Song, C. Cui, Chem. Commun. 2006, 1763–1765;
- 3hM. R. Mason, B. Song, Y. Han, X. Hu, Inorg. Chim. Acta 2008, 361, 3332–3337;
- 3iX. Wang, Z. Zhu, Y. Peng, H. Lei, J. C. Fettinger, P. P. Power, J. Am. Chem. Soc. 2009, 131, 6912–6913;
- 3jA. Fukazawa, J. L. Dutton, C. Fan, L. G. Mercier, A. Y. Houghton, Q. Wu, W. E. Piers, M. Parvez, Chem. Sci. 2012, 3, 1814–1818;
- 3kM. J. Cowley, Y. Ohmori, V. Huch, M. Ichinohe, A. Sekiguchi, D. Scheschkewitz, Angew. Chem. Int. Ed. 2013, 52, 13247–13250; Angew. Chem. 2013, 125, 13489–13492;
- 3lH. Braunschweig, T. Dellermann, R. D. Dewhurst, W. C. Ewing, K. Hammond, J. O. C. Jimenez-Halla, T. Kramer, I. Krummenacher, J. Mies, A. K. Phukan, A. Vargas, Nat. Chem. 2013, 5, 1025–1028;
- 3mM. Sajid, L.-M. Elmer, C. Rosorius, C. G. Daniliuc, S. Grimme, G. Kehr, G. Erker, Angew. Chem. Int. Ed. 2013, 52, 2243–2246; Angew. Chem. 2013, 125, 2299–2302;
- 3nM. Sajid, A. Lawzer, W. Dong, C. Rosorius, W. Sander, B. Schirmer, S. Grimme, C. G. Daniliuc, G. Kehr, G. Erker, J. Am. Chem. Soc. 2013, 135, 18567–18574;
- 3oM. Sajid, G. Kehr, C. G. Daniliuc, G. Erker, Angew. Chem. Int. Ed. 2014, 53, 1118–1121; Angew. Chem. 2014, 126, 1136–1139;
- 3pH. Braunschweig, R. D. Dewhurst, F. Hupp, M. Nutz, K. Radacki, C. W. Tate, A. Vargas, Q. Ye, Nature 2015, 522, 327–330;
- 3qW. Lu, H. Hu, Y. Li, R. Ganguly, R. Kinjo, J. Am. Chem. Soc. 2016, 138, 6650–6661;
- 3rB. Wang, G. Luo, M. Nishiura, Y. Luo, Z. Hou, J. Am. Chem. Soc. 2017, 139, 16967–16973;
- 3sX. Shi, C. Hou, C. Zhou, Y. Song, J. Cheng, Angew. Chem. Int. Ed. 2017, 56, 16650–16653; Angew. Chem. 2017, 129, 16877–16880.
- 4
- 4aR. Dobrovetsky, D. W. Stephan, J. Am. Chem. Soc. 2013, 135, 4974–4977;
- 4bL. D. Curless, E. R. Clark, J. Cid, A. Del Grosso, M. J. Ingleson, Chem. Commun. 2015, 51, 10903–10906;
- 4cM. Majumdar, I. Omlor, C. B. Yildiz, A. Azizoglu, V. Huch, D. Scheschkewitz, Angew. Chem. Int. Ed. 2015, 54, 8746–8750; Angew. Chem. 2015, 127, 8870–8874;
- 4dM. Arrowsmith, J. Böhnke, H. Braunschweig, M. A. Celik, Angew. Chem. Int. Ed. 2017, 56, 14287–14292; Angew. Chem. 2017, 129, 14475–14480;
- 4eM. Devillard, B. de Bruin, M. A. Siegler, J. I. van der Vlugt, Chem. Eur. J. 2017, 23, 13628–13632;
- 4fC. B. Yildiz, D. Scheschkewitz, Organometallics 2017, 36, 3035–3042.
- 5
- 5aS. Ozaki, Chem. Rev. 1972, 72, 457–496;
- 5bA. Dömling, Chem. Rev. 2006, 106, 17–89;
- 5cG. Qiu, Q. Ding, J. Wu, Chem. Soc. Rev. 2013, 42, 5257–5269;
- 5dV. P. Boyarskiy, N. A. Bokach, K. V. Luzyanin, V. Y. Kukushkin, Chem. Rev. 2015, 115, 2698–2779;
- 5eB. Song, B. Xu, Chem. Soc. Rev. 2017, 46, 1103–1123.
- 6
- 6aF. Bauer, H. Braunschweig, K. Schwab, Organometallics 2010, 29, 934–938;
- 6bO. Ekkert, G. Kehr, C. G. Daniliuc, R. Fröhlich, B. Wibbeling, J. L. Petersen, G. Erker, Z. Anorg. Allg. Chem. 2013, 639, 2455–2462;
- 6cO. Ekkert, G. G. Miera, T. Wiegand, H. Eckert, B. Schirmer, J. L. Petersen, C. G. Daniliuc, R. Frohlich, S. Grimme, G. Kehr, G. Erker, Chem. Sci. 2013, 4, 2657–2664;
- 6dZ. D. Brown, P. P. Power, Inorg. Chem. 2013, 52, 6248–6259;
- 6eN. Kielland, E. Vicente-García, M. Revés, N. Isambert, M. J. Arévalo, R. Lavilla, Adv. Synth. Catal. 2013, 355, 3273–3284;
- 6fB. R. Barnett, C. E. Moore, A. L. Rheingold, J. S. Figueroa, Chem. Commun. 2015, 51, 541–544;
- 6gA. Jana, V. Huch, H. S. Rzepa, D. Scheschkewitz, Angew. Chem. Int. Ed. 2015, 54, 289–292; Angew. Chem. 2015, 127, 291–295;
- 6hL. Kong, W. Lu, Y. Li, R. Ganguly, R. Kinjo, Angew. Chem. Int. Ed. 2016, 55, 14718–14722; Angew. Chem. 2016, 128, 14938–14942;
- 6iA. C. McQuilken, Q. M. Dao, A. J. P. Cardenas, J. A. Bertke, S. Grimme, T. H. Warren, Angew. Chem. Int. Ed. 2016, 55, 14335–14339; Angew. Chem. 2016, 128, 14547–14551;
- 6jJ. Li, B. Li, R. Liu, L. Jiang, H. Zhu, H. W. Roesky, S. Dutta, D. Koley, W. Liu, Q. Ye, Chem. Eur. J. 2016, 22, 14499–14503;
- 6kA. K. Adhikari, M. B. Sárosi, T. Grell, P. Lönnecke, E. Hey-Hawkins, Chem. Eur. J. 2016, 22, 15664–15668;
- 6lL. Kong, W. Lu, Y. Li, R. Ganguly, R. Kinjo, J. Am. Chem. Soc. 2016, 138, 8623–8629;
- 6mW. Lu, Y. Li, R. Ganguly, R. Kinjo, Angew. Chem. Int. Ed. 2017, 56, 9829–9832; Angew. Chem. 2017, 129, 9961–9964.
- 7
- 7aT. Ishiyama, N. Matsuda, N. Miyaura, A. Suzuki, J. Am. Chem. Soc. 1993, 115, 11018–11019;
- 7bR. T. Baker, P. Nguyen, T. B. Marder, S. A. Westcott, Angew. Chem. Int. Ed. Engl. 1995, 34, 1336–1338; Angew. Chem. 1995, 107, 1451–1452;
- 7cT. Ishiyama, M. Yamamoto, N. Miyaura, Chem. Commun. 1997, 689–690;
- 7dH. Y. Chen, S. Schlecht, T. C. Semple, J. F. Hartwig, Science 2000, 287, 1995–1997;
- 7eT. Ishiyama, J. Takagi, K. Ishida, N. Miyaura, N. R. Anastasi, J. F. Hartwig, J. Am. Chem. Soc. 2002, 124, 390–391;
- 7fT. Ishiyama, N. Miyaura, Chem. Rec. 2004, 3, 271–280;
- 7gT. Ishiyama, N. Miyaura, Pure Appl. Chem. 2006, 78, 1369–1375;
- 7hI. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy, J. F. Hartwig, Chem. Rev. 2010, 110, 890–931.
- 8H. Asakawa, K.-H. Lee, Z. Lin, M. Yamashita, Nat. Commun. 2014, 5, 4245.
- 9C. Kojima, K.-H. Lee, Z. Lin, M. Yamashita, J. Am. Chem. Soc. 2016, 138, 6662–6669.
- 10Y. Katsuma, H. Asakawa, K.-H. Lee, Z. Lin, M. Yamashita, Organometallics 2016, 35, 2563–2566.
- 11Y. Katsuma, H. Asakawa, M. Yamashita, Chem. Sci. 2018, 9, 1301–1310..
- 12H. Asakawa, K.-H. Lee, K. Furukawa, Z. Lin, M. Yamashita, Chem. Eur. J. 2015, 21, 4267–4271.
- 13
- 13aT. Wartik, R. Moore, H. I. Schlesinger, J. Am. Chem. Soc. 1949, 71, 3265–3266;
- 13bG. Urry, J. Kerrigan, T. D. Parsons, H. I. Schlesinger, J. Am. Chem. Soc. 1954, 76, 5299–5301;
- 13cG. Urry, T. Wartik, R. E. Moore, H. I. Schlesinger, J. Am. Chem. Soc. 1954, 76, 5293–5298;
- 13dA. Finch, H. I. Schlesinger, J. Am. Chem. Soc. 1958, 80, 3573–3574;
- 13eP. Ceron, A. Finch, J. Frey, J. Kerrigan, T. Parsons, G. Urry, H. I. Schlesinger, J. Am. Chem. Soc. 1959, 81, 6368–6371;
- 13fA. Höfner, B. Ziegler, R. Hunold, P. Willershausen, W. Massa, A. Berndt, Angew. Chem. Int. Ed. Engl. 1991, 30, 594–596; Angew. Chem. 1991, 103, 580–582;
- 13gA. Moezzi, M. M. Olmstead, R. A. Bartlett, P. P. Power, Organometallics 1992, 11, 2383–2388;
- 13hA. Moezzi, M. M. Olmstead, P. P. Power, J. Am. Chem. Soc. 1992, 114, 2715–2717;
- 13iP. P. Power, Inorg. Chim. Acta 1992, 198–200, 443–447;
- 13jW. J. Grigsby, P. Power, Chem. Eur. J. 1997, 3, 368–375;
- 13kH. Hommer, H. Noth, J. Knizek, W. Ponikwar, H. Schwenk-Kircher, Eur. J. Inorg. Chem. 1998, 1519–1527;
10.1002/(SICI)1099-0682(199810)1998:10<1519::AID-EJIC1519>3.0.CO;2-# CAS Web of Science® Google Scholar
- 13lH. Braunschweig, A. Damme, R. D. Dewhurst, T. Kramer, T. Kupfer, K. Radacki, E. Siedler, A. Trumpp, K. Wagner, C. Werner, J. Am. Chem. Soc. 2013, 135, 8702–8707;
- 13mH. Braunschweig, A. Damme, T. Kupfer, Chem. Commun. 2013, 49, 2774–2776;
- 13nN. Arnold, H. Braunschweig, A. Damme, R. D. Dewhurst, L. Pentecost, K. Radacki, S. Stellwag-Konertz, T. Thiess, A. Trumpp, A. Vargas, Chem. Commun. 2016, 52, 4898–4901;
- 13oM. Arrowsmith, J. Bohnke, H. Braunschweig, Dei, R. D. Dewhurst, W. C. Ewing, C. Horl, J. Mies, J. H. Muessig, Chem. Commun. 2017, 53, 8265–8267.
- 14N. Tsukahara, H. Asakawa, K.-H. Lee, Z. Lin, M. Yamashita, J. Am. Chem. Soc. 2017, 139, 2593–2596.
- 15E. J. Corey, T. Shibata, T. W. Lee, J. Am. Chem. Soc. 2002, 124, 3808–3809.
- 16The C(sp3)−H coupling constants in cyclic hexose derivatives were reported to be 1JCH=130–140 Hz with ideal sp3 hybridized carbon atom owing to the six-membered ring. See: T. Klepach, W. Zhang, I. Carmichael, A. S. Serianni, J. Org. Chem. 2008, 73, 4376–4387. A slightly distorted five-membered ring in 2 would affect to the hybridization of C8 to possess smaller bond angle of B1-C8-C15=102.92(11)° than that of ideal sp3 carbon. It would be expected that this small B-C-C angle would lead to increased s-character of the C−H bond and make coupling constant smaller.
- 17R. T. Williamson, A. V. Buevich, G. E. Martin, Org. Lett. 2012, 14, 5098–5101.
- 18T. D. W. Claridge, High-Resolution NMR Techniques in Organic Chemistry, Elsevier Science, Amsterdam, 2016.
- 19Related Pt–oxoborane species were isolated, characterized, and shown to be dimerized. See:
- 19aH. Braunschweig, K. Radacki, A. Schneider, Science 2010, 328, 345–347;
- 19bH. Braunschweig, K. Radacki, A. Schneider, Angew. Chem. Int. Ed. 2010, 49, 5993–5996; Angew. Chem. 2010, 122, 6130–6133.
- 20The arene–boraalkene complex 13 could be distinguished from pyridine-stabilized 7-borabicyclo[4.1.0]hepta-3,5-diene derivatives because of the longer B−C bond in 13. See:
- 20aY.-L. Rao, H. Amarne, S.-B. Zhao, T. M. McCormick, S. Martić, Y. Sun, R.-Y. Wang, S. Wang, J. Am. Chem. Soc. 2008, 130, 12898–12900;
- 20bH. Amarne, C. Baik, S. K. Murphy, S. Wang, Chem. Eur. J. 2010, 16, 4750–4761;
- 20cH. Amarne, C. Baik, R.-Y. Wang, S. Wang, Organometallics 2011, 30, 665–668;
- 20dY.-L. Rao, H. Amarne, S. Wang, Coord. Chem. Rev. 2012, 256, 759–770;
- 20eY.-L. Rao, L. D. Chen, N. J. Mosey, S. Wang, J. Am. Chem. Soc. 2012, 134, 11026–11034;
- 20fY.-L. Rao, H. Amarne, L. D. Chen, M. L. Brown, N. J. Mosey, S. Wang, J. Am. Chem. Soc. 2013, 135, 3407–3410;
- 20gY. L. Rao, C. Hörl, H. Braunschweig, S. Wang, Angew. Chem. Int. Ed. 2014, 53, 9086–9089; Angew. Chem. 2014, 126, 9232–9236;
- 20hS. K. Mellerup, L. Häfele, A. Lorbach, X. Wang, S. Wang, Org. Lett. 2017, 19, 3851–3854.
- 21The difference in steric factor between tBu and Xyl contributed to alter the lower-energy pathways from 21 to 16 or 22. See the Supporting Information for details of the DFT calculations.
- 22CCDC 1817085 (2), 1817086 (16), 1817087 (17), and 1817088 (18) contain the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.