Dynamic Kinetic Resolution of Heterobiaryl Ketones by Zinc-Catalyzed Asymmetric Hydrosilylation
Corresponding Author
Dr. Valentín Hornillos
Instituto Investigaciones Químicas (CSIC-US), Centro de Innovación en Química Avanzada (ORFEO-CINQA), C/ Américo Vespucio 49, 41092 Sevilla, Spain
Search for more papers by this authorJosé A. Carmona
Instituto Investigaciones Químicas (CSIC-US), Centro de Innovación en Química Avanzada (ORFEO-CINQA), C/ Américo Vespucio 49, 41092 Sevilla, Spain
Search for more papers by this authorDr. Abel Ros
Instituto Investigaciones Químicas (CSIC-US), Centro de Innovación en Química Avanzada (ORFEO-CINQA), C/ Américo Vespucio 49, 41092 Sevilla, Spain
Departamento de Química Orgánica (Universidad de Sevilla), Centro de Innovación en Química Avanzada (ORFEO-CINQA), C/ Prof. García González 1, 41012 Sevilla, Spain
Search for more papers by this authorDr. Javier Iglesias-Sigüenza
Departamento de Química Orgánica (Universidad de Sevilla), Centro de Innovación en Química Avanzada (ORFEO-CINQA), C/ Prof. García González 1, 41012 Sevilla, Spain
Search for more papers by this authorDr. Joaquín López-Serrano
Departamento de Química Inorgánica and Centro de Innovación en Química Avanzada (ORFEO-CINQA)., Universidad de Sevilla and Instituto de Investigaciones Científicas (CSIC-US), Avda. Américo Vespucio 49, 41092 Sevilla, Spain
Search for more papers by this authorCorresponding Author
Prof. Rosario Fernández
Departamento de Química Orgánica (Universidad de Sevilla), Centro de Innovación en Química Avanzada (ORFEO-CINQA), C/ Prof. García González 1, 41012 Sevilla, Spain
Search for more papers by this authorCorresponding Author
Prof. José M. Lassaletta
Instituto Investigaciones Químicas (CSIC-US), Centro de Innovación en Química Avanzada (ORFEO-CINQA), C/ Américo Vespucio 49, 41092 Sevilla, Spain
Search for more papers by this authorCorresponding Author
Dr. Valentín Hornillos
Instituto Investigaciones Químicas (CSIC-US), Centro de Innovación en Química Avanzada (ORFEO-CINQA), C/ Américo Vespucio 49, 41092 Sevilla, Spain
Search for more papers by this authorJosé A. Carmona
Instituto Investigaciones Químicas (CSIC-US), Centro de Innovación en Química Avanzada (ORFEO-CINQA), C/ Américo Vespucio 49, 41092 Sevilla, Spain
Search for more papers by this authorDr. Abel Ros
Instituto Investigaciones Químicas (CSIC-US), Centro de Innovación en Química Avanzada (ORFEO-CINQA), C/ Américo Vespucio 49, 41092 Sevilla, Spain
Departamento de Química Orgánica (Universidad de Sevilla), Centro de Innovación en Química Avanzada (ORFEO-CINQA), C/ Prof. García González 1, 41012 Sevilla, Spain
Search for more papers by this authorDr. Javier Iglesias-Sigüenza
Departamento de Química Orgánica (Universidad de Sevilla), Centro de Innovación en Química Avanzada (ORFEO-CINQA), C/ Prof. García González 1, 41012 Sevilla, Spain
Search for more papers by this authorDr. Joaquín López-Serrano
Departamento de Química Inorgánica and Centro de Innovación en Química Avanzada (ORFEO-CINQA)., Universidad de Sevilla and Instituto de Investigaciones Científicas (CSIC-US), Avda. Américo Vespucio 49, 41092 Sevilla, Spain
Search for more papers by this authorCorresponding Author
Prof. Rosario Fernández
Departamento de Química Orgánica (Universidad de Sevilla), Centro de Innovación en Química Avanzada (ORFEO-CINQA), C/ Prof. García González 1, 41012 Sevilla, Spain
Search for more papers by this authorCorresponding Author
Prof. José M. Lassaletta
Instituto Investigaciones Químicas (CSIC-US), Centro de Innovación en Química Avanzada (ORFEO-CINQA), C/ Américo Vespucio 49, 41092 Sevilla, Spain
Search for more papers by this authorDedicated to Professor A. Ulises Acuña
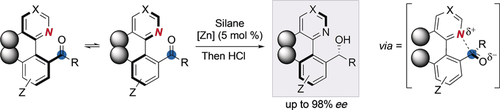
Graphical Abstract
The dynamic duo: A nitrogen atom and the carbonyl group in heterobiaryl ketones form a Lewis pair which is responsible for the labilization of the stereogenic axis, and constitutes the key strategy for developing a zinc-catalyzed asymmetric hydrosilylation by dynamic kinetic resolution. This process simultaneously installs a stereogenic axis and a stereocenter for the highly enantioselective synthesis of heterobiaryl carbinols.
Abstract
A diastereo- and highly enantioselective dynamic kinetic resolution (DKR) of configurationally labile heterobiaryl ketones is described. The DKR proceeds by zinc-catalyzed hydrosilylation of the carbonyl group, thus leading to secondary alcohols bearing axial and central chirality. The strategy relies on the labilization of the stereogenic axis that takes place thanks to a Lewis acid–base interaction between a nitrogen atom in the heterocycle and the ketone carbonyl group. The synthetic utility of the methodology is demonstrated through stereospecific transformations into either N,N-ligands or appealing axially chiral, bifunctional thiourea organocatalysts.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201713200-sup-0001-misc_information.pdf9.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. Bringmann, T. Gulder, T. A. M. Gulder, M. Breuning, Chem. Rev. 2011, 111, 563;
- 1bM. C. Kozlowski, B. J. Morgan, E. C. Linton, Chem. Soc. Rev. 2009, 38, 3193;
- 1cJ. E. Smyth, N. M. Butler, P. A. Keller, Nat. Prod. Rep. 2015, 32, 1562.
- 2For reviews, see:
- 2aY. Chen, S. Yekta, A. K. Yudin, Chem. Rev. 2003, 103, 3155;
- 2bH. Shimizu, I. Nagasaki, T. Saito, Tetrahedron 2005, 61, 5405;
- 2cY.-M. Li, F.-Y. Kwong, W.-Y. Yu, A. S. C. Chan, Coord. Chem. Rev. 2007, 251, 2119;
- 2dT. Akiyama, Chem. Rev. 2007, 107, 5744;
- 2eY. Canac, R. Chauvin, Eur. J. Inorg. Chem. 2010, 2325;
- 2fD. Parmar, E. Sugiono, S. Raja, M. Rueping, Chem. Rev. 2014, 114, 9047;
- 2gS. Kaneko, Y. Kumatabara, S. Shirakawa, Org. Biomol. Chem. 2016, 14, 5367.
- 3
- 3aD. Zhang, Q. Wang, Coord. Chem. Rev. 2015, 286, 1;
- 3bJ. Wencel-Delord, A. Panossian, F. R. Leroux, F. Colobert, Chem. Soc. Rev. 2015, 44, 3418;
- 3cP. Loxq, E. Manoury, R. Poli, E. Deydier, A. Labande, Coord. Chem. Rev. 2016, 308, 131.
- 4
- 4aF. Kakiuchi, P. L. Gendre, A. Yamada, H. Ohtaki, S. Murai, Tetrahedron: Asymmetry 2000, 11, 2647;
- 4bJ. Zheng, S.-L. You, Angew. Chem. Int. Ed. 2014, 53, 13244; Angew. Chem. 2014, 126, 13460;
- 4cJ. Zheng, W.-J. Cui, C. Zheng, S.-L. You, J. Am. Chem. Soc. 2016, 138, 5242;
- 4dC. K. Hazra, Q. Dherbassy, J. Wencel-Delord, F. Colobert, Angew. Chem. Int. Ed. 2014, 53, 13871; Angew. Chem. 2014, 126, 14091;
- 4eQ. Dherbassy, G. Schwertz, M. Chessé, C. K. Hazra, J. Wencel-Delord, F. Colobert, Chem. Eur. J. 2016, 22, 1735;
- 4fY.-N. Ma, H.-Y. Zhang, S.-D. Yang, Org. Lett. 2015, 17, 2034;
- 4gQ.-J. Yao, S. Zhang, B.-B. Zhan, B.-F. Shi, Angew. Chem. Int. Ed. 2017, 56, 6617; Angew. Chem. 2017, 129, 6717.
- 5
- 5aK. Tanaka, Chem. Asian J. 2009, 4, 508;
- 5bM. Amatore, C. Auber, Eur. J. Org. Chem. 2015, 265;
- 5cK. Tanaka, G. Nishida, A. Wada, K. Noguchi, Angew. Chem. Int. Ed. 2004, 43, 3795; Angew. Chem. 2004, 116, 3883.
- 6Seminal report:
- 6aJ. Gustafson, D. Lim, S. J. Miller, Science 2010, 328, 1251; For recent reviews see:
- 6bP. Renzi, Org. Biomol. Chem. 2017, 15, 4506;
- 6cR. M. Witzig, D. Lotter, V. C. Fäseke, C. Sparr, Chem. Eur. J. 2017, 23, 12960;
- 6dG. Bencivenni, Synlett 2015, 26, 1915.
- 7
- 7aG. Bringmann, T. Hartung, Angew. Chem. Int. Ed. Engl. 1992, 31, 761; Angew. Chem. 1992, 104, 782;
- 7bG. Bringmann, J. Kraus, M. Breuning, S. Tasler, Synthesis 1999, 525;
- 7cG. Bringmann, S. Tasler, R.-M. Pfeifer, M. Breuning, J. Organomet. Chem. 2002, 661, 49;
- 7dG. Bringmann, M. Breuning, P. Henschel, J. Hinrichs, Org. Synth. 2002, 79, 72.
- 8T. Ashizawa, S. Tanaka, T. Yamada, Org. Lett. 2008, 10, 2521.
- 9C. Yu, H. Huang, Y. Zhang, W. Wang, J. Am. Chem. Soc. 2016, 138, 6956.
- 10K. Mori, T. Itakura, T. Akiyama, Angew. Chem. Int. Ed. 2016, 55, 11642; Angew. Chem. 2016, 128, 11814.
- 11J. Zhang, J. Wang, Angew. Chem. Int. Ed. 2018, 57, 465; Angew. Chem. 2018, 130, 474.
- 12S. Staniland, R. W. Adams, J. J. W. McDouall, I. Maffucci, A. Contini, D. M. Grainger, N. J. Turner, J. Clayden, Angew. Chem. Int. Ed. 2016, 55, 10755; Angew. Chem. 2016, 128, 10913.
- 13
- 13aA. Ros, B. Estepa, P. Ramírez-López, E. Álvarez, R. Fernández, J. M. Lassaletta, J. Am. Chem. Soc. 2013, 135, 15730;
- 13bP. Ramírez-López, A. Ros, B. Estepa, R. Fernández, B. Fiser, E. Gómez-Bengoa, J. M. Lassaletta, ACS Catal. 2016, 6, 3955;
- 13cSee also: V. Bhat, S. Wang, B. M. Stoltz, S. C. Virgil, J. Am. Chem. Soc. 2013, 135, 16829;
- 13dP. Ramírez-López, A. Ros, A. Romero-Arenas, J. Iglesias-Sigüenza, R. Fernández, J. M. Lassaletta, J. Am. Chem. Soc. 2016, 138, 12053;
- 13eV. Hornillos, A. Ros, P. Ramirez-López, J. Iglesias-Sigüenza, R. Fernández, J. M. Lassaletta, Chem. Commun. 2016, 52, 14121.
- 14
- 14a Hydrosilylation: A Comprehensive Review on Recent Advances (Ed.: ), Springer, Dordrecht, 2009;
- 14bS. Díez-González, P. S. Nolan, Org. Prep. Proced. Int. 2007, 39, 523.
- 15Seminal work:
- 15aH. Mimoun, J. Y. De Saint Laumer, L. Giannini, R. Scopelliti, C. Floriani, J. Am. Chem. Soc. 1999, 121, 6158;
- 15bN. Shaikh, K. Junge, S. Enthaler, M. Beller, Angew. Chem. Int. Ed. 2008, 47, 2497; Angew. Chem. 2008, 120, 2531; Reviews:
- 15cY. Li, K. Junge, M. Beller in Zinc Catalysis: Applications in Organic Synthesis (Eds.: ), Wiley-VCH, Weinheim, 2015, pp. 14–19.
- 16CCDC 1589898, 1589899, 1589900 and 1813056 [2 a, (Ra,R)-3 a, (Ra,S)-7, and (R,R)-3′′e] contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 17The compound 4 was synthesized by a dynamic kinetic Heck reaction between rac-1 a and butyl vinyl ether: J. A. Carmona, V. Hornillos, P. Ramírez-López, A. Ros, J. Iglesias-Sigüenza, R. Fernández, J. M. Lassaletta, Submitted.
- 18For the sake of comparison, Relaxed Potential Energy Scan (PES) suggest a barrier of more than 35 kcal mol−1 for the rotation of the 3-methyl-pyridyl and naphthyl moieties of the corresponding alcohol 3 c around the C−C axis (see the Supporting Information).
- 19For selected examples using Zn(OAc)2 and chiral diamine ligands, see:
- 19bT. Inagaki, Y. Yamada, L. T. Phong, A. Furuta, J.-I. Ito, H. Nishiyama, Synlett 2009, 253;
- 19cS. Pang, J. Peng, J. Li, Y. Bai, W. Xiao, G. Lai, Chirality 2013, 25, 275;
- 19dM. Szewczyk, F. Stanek, A. Bezłada, J. Mlynarskia, Adv. Synth. Catal. 2015, 357, 3727;
- 19eK. Junge, K. Möller, B. Wendt, S. Das, D. Gördes, K. Thurow, M. Beller, Chem. Asian J. 2012, 7, 314; For a recent review see:
- 19aD. Łowicki, S. Baś, J. Mlynarski, Tetrahedron 2015, 71, 1339.
- 20Diphenylethylenediamine ligands were obtained in one-step by reaction of commercially available 1,2-diphenylethylenediamine with benzyl halides in a presence of potassium carbonate. S. Kobayashi, R. Matsubara, Y. Nakamura, H. Kitagawa, M. Sugiura, J. Am. Chem. Soc. 2003, 125, 2507.
- 21Treatment of (Sa,R)-3 a with mesyl chloride and Et3N in CH2Cl2 at 40 °C afforded (Sa)1-(2-vinylnaphthalen-1-yl)isoquinoline, whose configuration was assigned by comparison with literature data (See Ref. [13e]).
- 22
- 22aS. B. Cortright, J. N. Johnston, Angew. Chem. Int. Ed. 2002, 41, 345;
10.1002/1521-3773(20020118)41:2<345::AID-ANIE345>3.0.CO;2-U CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 355;
- 22bS. B. Cortright, J. C. Huffman, R. A. Yoder, J. N. Coalter, J. N. Johnston, Organometallics 2004, 23, 2238;
- 22cS. B. Luesse, C. M. Counceller, J. C. Wilt, B. R. Perkins, J. N. Johnston, Org. Lett. 2008, 10, 2445.