Iodine(III) Derivatives as Halogen Bonding Organocatalysts
Flemming Heinen
Fakultät für Chemie und Biochemie, Ruhr-Universität Bochum, Universitätsstraße 150, 44801 Bochum, Germany
These authors contributed equally to this work.
Search for more papers by this authorElric Engelage
Fakultät für Chemie und Biochemie, Ruhr-Universität Bochum, Universitätsstraße 150, 44801 Bochum, Germany
These authors contributed equally to this work.
Search for more papers by this authorAlexander Dreger
Fakultät für Chemie und Biochemie, Ruhr-Universität Bochum, Universitätsstraße 150, 44801 Bochum, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Robert Weiss
Institut für Organische Chemie, Friedrich-Alexander-Universität Erlangen-Nürnberg, Henkestraße 42, 91054 Erlangen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Stefan M. Huber
Fakultät für Chemie und Biochemie, Ruhr-Universität Bochum, Universitätsstraße 150, 44801 Bochum, Germany
Search for more papers by this authorFlemming Heinen
Fakultät für Chemie und Biochemie, Ruhr-Universität Bochum, Universitätsstraße 150, 44801 Bochum, Germany
These authors contributed equally to this work.
Search for more papers by this authorElric Engelage
Fakultät für Chemie und Biochemie, Ruhr-Universität Bochum, Universitätsstraße 150, 44801 Bochum, Germany
These authors contributed equally to this work.
Search for more papers by this authorAlexander Dreger
Fakultät für Chemie und Biochemie, Ruhr-Universität Bochum, Universitätsstraße 150, 44801 Bochum, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Robert Weiss
Institut für Organische Chemie, Friedrich-Alexander-Universität Erlangen-Nürnberg, Henkestraße 42, 91054 Erlangen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Stefan M. Huber
Fakultät für Chemie und Biochemie, Ruhr-Universität Bochum, Universitätsstraße 150, 44801 Bochum, Germany
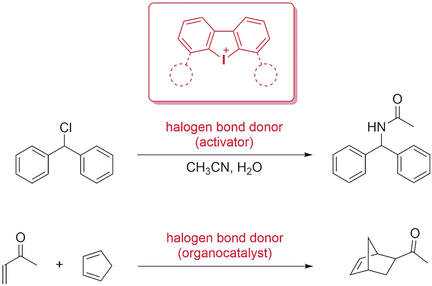
Search for more papers by this authorGraphical Abstract
Activation with iodine: Hypervalent iodine(III) compounds were used as activators or organocatalysts in benchmark reactions. The crucial role of halogen bonding in these processes was demonstrated for the first time by a series of comparative experiments, including with sterically blocked derivatives. The activities of these monodentate Lewis acids match or exceed those of previously used bidentate cationic halogen bond donors.
Abstract
Hypervalent iodine(III) derivatives are known as versatile reagents in organic synthesis, but there is only one previous report on their use as Lewis acidic organocatalysts. Herein, we present first strong indications for the crucial role of halogen bonding in this kind of catalyses. To this end, the solvolysis of benzhydryl chloride and the Diels–Alder reaction of cyclopentadiene with methyl vinyl ketone served as benchmark reactions for halide abstraction and the activation of neutral compounds. Iodolium compounds (cyclic diaryl iodonium species) were used as activators or catalysts, and we were able to markedly reduce or completely switch off their activity by sterically blocking one or two of their electrophilic axes. Compared with previously established bidentate cationic halogen bond donors, the monodentate organoiodine derivatives used herein are at least similarly active (in the Diels–Alder reaction) or even decidedly more active (in benzhydryl chloride solvolysis).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201713012-sup-0001-misc_information.pdf2.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. Wirth, Hypervalent Iodine Chemistry, 1st ed., Springer, Berlin, Heidelberg, 2003;
10.1007/3-540-46114-0 Google Scholar
- 1bA. Yoshimura, V. V. Zhdankin, Chem. Rev. 2016, 116, 3328;
- 1c“Hypervalent Iodine”: T. Dohi, Y. Kita in Iodine Chemistry and Applications (Ed.: ), Wiley, Hoboken, 2014, pp. 103–157.
10.1002/9781118909911.ch7 Google Scholar
- 2E. A. Merritt, B. Olofsson, Angew. Chem. Int. Ed. 2009, 48, 9052; Angew. Chem. 2009, 121, 9214.
- 3
- 3aA. P. Antonchick, S. Manna, R. Narayan, Synlett 2015, 26, 1785;
- 3bM. Uyanik, T. Yasui, K. Ishihara, Angew. Chem. Int. Ed. 2010, 49, 2175; Angew. Chem. 2010, 122, 2221;
- 3cV. V. Zhdankin, P. J. Stang, Chem. Rev. 2008, 108, 5299.
- 4
- 4aF. V. Singh, T. Wirth, Chem. Asian J. 2014, 9, 950;
- 4bR. D. Richardson, T. Wirth, Angew. Chem. Int. Ed. 2006, 45, 4402; Angew. Chem. 2006, 118, 4510.
- 5
- 5aE. A. Merrit, B. Olofsson, Synthesis 2011, 4, 517;
- 5bS. V. Kohlhepp, T. Gulder, Chem. Soc. Rev. 2016, 45, 6270;
- 5c“Alkynylation with Hypervalent Iodine Reagents”: J. Waser in Hypervalent Iodine Chemistry, Vol. 373 (Ed.: ), Springer, Cham, 2016, pp. 187–222;
- 5dJ. Charpentier, N. Früh, A. Togni, Chem. Rev. 2015, 115, 650;
- 5eK. Kiyokawa, T. Kosaka, T. Kojima, S. Minakata, Angew. Chem. Int. Ed. 2015, 54, 13719; Angew. Chem. 2015, 127, 13923.
- 6Iodonium ylides are a notable exception; they are also neutral compounds, but carry only two substituents on the iodine center.
- 7G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati, G. Terraneo, Chem. Rev. 2016, 116, 2478.
- 8
- 8a“Halogen Bonding in Hypervalent Iodine Compounds”: L. Catalano, G. Cavallo, P. Metrangolo, G. Resnati in Hypervalent Chemistry, Vol. 373 of Topics in Current Chemistry (Ed.: ), Springer, Cham, 2016, pp. 289–309;
- 8bG. Cavallo, J. S. Murray, P. Politzer, T. Pilati, M. Ursini, G. Resnati, IUCrJ 2017, 4, 411.
- 9G. R. Desiraju, P. S. Ho, L. Kloo, A. C. Legon, R. Marquardt, P. Metrangolo, P. Politzer, G. Resnati, K. Rissanen, Pure Appl. Chem. 2013, 85, 1711.
- 10
- 10aD. Bulfield, S. M. Huber, Chem. Eur. J. 2016, 22, 14434;
- 10bJ.-P. Gliese, S. H. Jungbauer, S. M. Huber, Chem. Commun. 2017, 53, 12052;
- 10cS. H. Jungbauer, S. M. Huber, J. Am. Chem. Soc. 2015, 137, 12110.
- 11For a related computational study, see: H. P. de Magalhães, A. Togni, H. P. Lüthi, J. Org. Chem. 2017, 82, 11799; see also: O. Kirshenboim, S. Kozuch, J. Phys. Chem. A 2016, 120, 9431.
- 12Y. Zhang, J. Han, Z.-J. Liu, RSC Adv. 2015, 5, 25485. For the also highly relevant experimental determination of the Lewis acidity of iodine(III) compounds, see: A. Labattut, P.-L. Tremblay, O. Moutounet, C. Y. Legault, J. Org. Chem. 2017, 82, 11891.
- 13
- 13aL. Mascarelli, G. Benati, Gazz. Chim. Ital. 1908, 38, 619;
- 13bJ. Collette, D. McGreer, R. Crawford, F. Chubb, R. B. Sandin, J. Am. Chem. Soc. 1956, 78, 3819;
- 13cR. C. Reynold, C. Fuson, R. L. Albright, J. Am. Chem. Soc. 1959, 81, 487;
- 13dP. S. Postnikov, O. A. Guselnikova, M. S. Yusubov, A. Yoshimura, V. N. Nemykin, V. V. Zhdankin, J. Org. Chem. 2015, 80, 5783;
- 13eH. Xie, S. Yang, C. Zhang, M. Ding, M. Liu, J. Guo, F. Zhang, J. Org. Chem. 2017, 82, 5250.
- 14D. Hellwinkel, G. Reiff, V. Nykodym, Liebigs Ann. Chem. 1977, 1013.
- 15H. Tomori, J. M. Fox, S. L. Buchwald, J. Org. Chem. 2000, 65, 5334.
- 16M. Bielawski, M. Zhu, B. Olofsson, Adv. Synth. Catal. 2007, 349, 2610.
- 17N. A. Yakelis, R. G. Bergman, Organometallics 2005, 24, 3579, and references cited therein.
- 18The corresponding structure of the chloride salt shows a similar coordination pattern; see Ref. [8b].
- 19A. Bondi, J. Phys. Chem. 1964, 68, 441.
- 20S. M. Walter, F. Kniep, L. Rout, F. P. Schmidtchen, E. Herdtweck, S. M. Huber, J. Am. Chem. Soc. 2012, 134, 8507.
- 21S. M. Walter, F. Kniep, E. Herdtweck, S. M. Huber, Angew. Chem. Int. Ed. 2011, 50, 7187; Angew. Chem. 2011, 123, 7325.
- 22S. H. Jungbauer, S. M. Walter, S. Schindler, L. Rout, F. Kniep, S. M. Huber, Chem. Commun. 2014, 50, 6281.
- 23Interestingly, the methylated compound 3 a is more active than the non-methylated one (1 a) in this reaction, for currently unknown reasons.
- 24D. von der Heiden, S. Bozkus, M. Klussmann, M. Breugst, J. Org. Chem. 2017, 82, 4037.
- 25The performance of catalyst 5 b was better in this study than in the originally published work (Ref. [21]), possibly owing to traces of triflate in the originally employed catalyst.
- 26CYLview, 1.0b; C. Y. Legault, Université de Sherbrooke 2009 (http://www.cylview.org).
- 27In the crystal structures of Figure 3, only weak contacts between the halide and suitable hydrogen-bond-donating substituents of the iodolium compounds were found (with distances of at least 95 % of the sum of the van der Waals radii).