Total Synthesis and Conformational Study of Callyaerin A: Anti-Tubercular Cyclic Peptide Bearing a Rare Rigidifying (Z)-2,3- Diaminoacrylamide Moiety
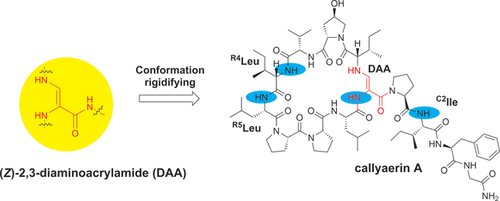
Graphical Abstract
A rigid structure: Callyaerin A, an anti-tuberculosis macrocyclic peptide containing a rare (Z)-2,3-diaminoacrylamide moiety, was synthesized in high yield using an uncommon building block, Fmoc-formylglycine-diethylacetal. Variable-temperature NMR studies revealed the high conformational rigidity that the (Z)-2,3-diaminoacrylamide moiety confers on peptide structure, thus highlighting its potential as a novel structural element for conformation constraints.
Abstract
The first synthesis of the anti-TB cyclic peptide callyaerin A (1), containing a rare (Z)-2,3-diaminoacrylamide bridging motif, is reported. Fmoc-formylglycine-diethylacetal was used as a masked equivalent of formylglycine in the synthesis of the linear precursor to 1. Intramolecular cyclization between the formylglycine residue and the N-terminal amine in the linear peptide precursor afforded the macrocyclic natural product 1. Synthetic 1 possessed potent anti-TB activity (MIC100=32 μm) while its all-amide congener was inactive. Variable-temperature NMR studies of both the natural product and its all-amide analogue revealed the extraordinary rigidity imposed by this diaminoacrylamide unit on peptide conformation. The work reported herein pinpoints the intrinsic role that the (Z)-2,3-diaminoacrylamide moiety confers on peptide bioactivity.