Total Synthesis of Maoecrystal P: Application of a Strained Bicyclic Synthon
Fan Su
Key Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, Beijing National Laboratory for Molecular Science, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
These authors contributed equally.
Search for more papers by this authorYandong Lu
Key Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, Beijing National Laboratory for Molecular Science, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
These authors contributed equally.
Search for more papers by this authorLingran Kong
Key Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, Beijing National Laboratory for Molecular Science, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
Search for more papers by this authorJingjing Liu
Peking-Tsinghua Center for Life Sciences, Academy of Advanced Interdisciplinary Studies, Peking University, Beijing, 100871 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Tuoping Luo
Key Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, Beijing National Laboratory for Molecular Science, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
Peking-Tsinghua Center for Life Sciences, Academy of Advanced Interdisciplinary Studies, Peking University, Beijing, 100871 China
Search for more papers by this authorFan Su
Key Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, Beijing National Laboratory for Molecular Science, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
These authors contributed equally.
Search for more papers by this authorYandong Lu
Key Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, Beijing National Laboratory for Molecular Science, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
These authors contributed equally.
Search for more papers by this authorLingran Kong
Key Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, Beijing National Laboratory for Molecular Science, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
Search for more papers by this authorJingjing Liu
Peking-Tsinghua Center for Life Sciences, Academy of Advanced Interdisciplinary Studies, Peking University, Beijing, 100871 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Tuoping Luo
Key Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, Beijing National Laboratory for Molecular Science, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
Peking-Tsinghua Center for Life Sciences, Academy of Advanced Interdisciplinary Studies, Peking University, Beijing, 100871 China
Search for more papers by this authorGraphical Abstract
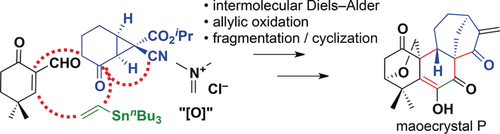
Less is not always more: A strategy involving the incorporation of a substituted cyclopropane ring to generate an overbred intermediate was devised for the total synthesis of the highly oxidized bioactive ent-kauranoid maoecrystal P. Starting from a strained bicyclo[4.1.0] ketone, a sequence based on intermolecular Diels–Alder cycloaddition, allylic oxidation, SmI2-mediated cyclization, and late-stage oxidation reactions led to the target molecule.
Abstract
A new strategy was devised for the total synthesis of highly oxidized ent-kauranoids. A highly regio- and diastereoselective intermolecular Diels–Alder cycloaddition involving a diene embedded in a substituted bicyclo[4.1.0] skeleton was used to assemble all carbon centers but C17 of the target molecule at an early stage of the synthesis. Subsequent synthetic steps, including redox manipulations, SmI2-mediated cyclization, and isomerization reactions, afforded the antitumor natural product maoecrystal P.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201711084-sup-0001-misc_information.pdf5.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aH.-D. Sun, S.-X. Huang, Q.-B. Han, Nat. Prod. Rep. 2006, 23, 673–698;
- 1bP. A. García, A. B. De Oliveira, R. Batista, Molecules 2007, 12, 455–483;
- 1cM. Liu, W.-G. Wang, H.-D. Sun, J.-X. Pu, Nat. Prod. Rep. 2017, 34, 1090–1140.
- 2For reviews of ent-kauranoid total synthesis, see:
- 2aK. E. Lazarski, B. J. Moritz, R. J. Thomson, Angew. Chem. Int. Ed. 2014, 53, 10588–10599; Angew. Chem. 2014, 126, 10762–10773;
- 2bP. S. Riehl, Y. C. DePorre, A. M. Armaly, E. J. Groso, C. S. Schindler, Tetrahedron 2015, 71, 6629–6650;
- 2cL. Zhu, S.-H. Huang, J. Yu, R. Hong, Tetrahedron Lett. 2015, 56, 23–31;
- 2dM.-J. Du, X.-G. Lei, Chin. J. Org. Chem. 2015, 35, 2447–2464.
- 3For syntheses of ent-kauranoids with an intact ent-kaurene carbon skeleton, see:
- 3aR. A. Bell, R. E. Ireland, R. A. Partyka, J. Org. Chem. 1962, 27, 3741–3744;
- 3bS. Masamune, J. Am. Chem. Soc. 1964, 86, 288–289;
- 3cR. A. Bell, R. E. Ireland, R. A. Partyka, J. Org. Chem. 1966, 31, 2530–2536;
- 3dR. E. Ireland, L. N. Mander, Tetrahedron Lett. 1965, 6, 2627–2632;
10.1016/S0040-4039(00)90219-3 Google Scholar
- 3eK. Mori, M. Matsui, Tetrahedron Lett. 1966, 7, 175–180;
10.1016/S0040-4039(00)70209-7 Google Scholar
- 3fK. Mori, M. Matsui, N. Ikekawa, Y. Sumiki, Tetrahedron Lett. 1966, 7, 3395–3400;
10.1016/S0040-4039(01)82800-8 Google Scholar
- 3gR. B. Turner, K. H. Ganshirt, P. E. Shaw, J. D. Tauber, J. Am. Chem. Soc. 1966, 88, 1776–1785;
- 3hK. Mori, M. Matsui, Tetrahedron 1966, 22, 879–884;
- 3iK. Mori, Y. Nakahara, M. Matsui, Tetrahedron Lett. 1970, 11, 2411–2414;
10.1016/S0040-4039(01)98242-5 Google Scholar
- 3jY. Nakahara, K. Mori, M. Matsui, Agric. Biol. Chem. 1971, 35, 918–928;
- 3kK. Mori, Y. Nakahara, M. Matsui, Tetrahedron 1972, 28, 3217–3226;
- 3lD. K. M. Duc, M. Fetizon, S. Lazare, J. Chem. Soc. Chem. Commun. 1975, 282;
10.1039/c39750000282 Google Scholar
- 3mF. E. Ziegler, J. A. Kloek, Tetrahedron 1977, 33, 373–380;
- 3nE. J. Corey, G. Wess, Y. B. Xiang, A. K. Singh, J. Am. Chem. Soc. 1987, 109, 4717–4718;
- 3oA. K. Singh, R. K. Bakshi, E. J. Corey, J. Am. Chem. Soc. 1987, 109, 6187–6189;
- 3pD. Backhaus, L. A. Paquette, Tetrahedron Lett. 1997, 38, 29–32;
- 3qE. J. Corey, K. Liu, J. Am. Chem. Soc. 1997, 119, 9929–9930;
- 3rE. C. Cherney, J. C. Green, P. S. Baran, Angew. Chem. Int. Ed. 2013, 52, 9019–9022; Angew. Chem. 2013, 125, 9189–9192;
- 3sJ. T. S. Yeoman, V. W. Mak, S. E. Reisman, J. Am. Chem. Soc. 2013, 135, 11764–11767;
- 3tJ. T. S. Yeoman, J. Y. Cha, V. W. Mak, S. E. Reisman, Tetrahedron 2014, 70, 4070–4088;
- 3uL. Zhu, J. Luo, R. Hong, Org. Lett. 2014, 16, 2162–2165;
- 3vX. Zhao, W. Li, J. Wang, D. Ma, J. Am. Chem. Soc. 2017, 139, 2932–2935;
- 3wC. He, J. Hu, Y. Wu, H. Ding, J. Am. Chem. Soc. 2017, 139, 6098–6101.
- 4
- 4aJ. Wang, Z. Lin, Q. Zhao, H. Sun, Phytochemistry 1998, 47, 307–309;
- 4bX.-M. Niu, S.-H. Li, S.-X. Mei, Z. Na, Q.-S. Zhao, Z.-W. Lin, H.-D. Sun, J. Nat. Prod. 2002, 65, 1892–1896;
- 4cW.-G. Wang, X.-N. Li, X. Du, H.-Y. Wu, X. Liu, J. Su, Y. Li, J.-X. Pu, H.-D. Sun, J. Nat. Prod. 2012, 75, 1102–1107.
- 5
- 5aC.-X. Liu, Q.-Q. Yin, H.-C. Zhou, Y.-L. Wu, J.-X. Pu, L. Xia, W. Liu, X. Huang, T. Jiang, M.-X. Wu, L.-C. He, Y.-X. Zhao, X.-L. Wang, W.-L. Xiao, H.-Z. Chen, Q. Zhao, A.-W. Zhou, L.-S. Wang, H.-D. Sun, G.-Q. Chen, Nat. Chem. Biol. 2012, 8, 486–493;
- 5bT. Zhen, C.-F. Wu, P. Liu, H.-Y. Wu, G.-B. Zhou, Y. Lu, J.-X. Liu, Y. Liang, K. K. Li, Y.-Y. Wang, Y.-Y. Xie, M.-M. He, H.-M. Cao, W.-N. Zhang, L.-M. Chen, K. Petrie, S.-J. Chen, Z. Chen, Sci. Transl. Med. 2012, 4, 127ra38;
- 5cL.-M. Kong, X. Deng, Z.-L. Zuo, H.-D. Sun, Q.-S. Zhao, Y. Li, Oncotarget 2014, 5, 11354–11364.
- 6
- 6aS. Zhao, J. Dai, M. Hu, C. Liu, R. Meng, X. Liu, C. Wang, T. Luo, Chem. Commun. 2016, 52, 4702–4705;
- 6bY. Guo, T. Quan, Y. Lu, T. Luo, J. Am. Chem. Soc. 2017, 139, 6815–6818.
- 7
- 7aE. Fujita, M. Shibuya, S. Nakamura, Y. Okada, T. Fujita, J. Chem. Soc. Perkin Trans. 1 1974, 165–177;
- 7bL. A. Paquette, D. Backhaus, R. Braun, T. L. Underiner, K. Fuchs, J. Am. Chem. Soc. 1997, 119, 9662–9671;
- 7cJ. Gong, G. Lin, W. Sun, C.-C. Li, Z. Yang, J. Am. Chem. Soc. 2010, 132, 16745–16746;
- 7dF. Peng, S. J. Danishefsky, J. Am. Chem. Soc. 2012, 134, 18860–18867;
- 7eP. Lu, Z. Gu, A. Zakarian, J. Am. Chem. Soc. 2013, 135, 14552–14555;
- 7fP. Lu, A. Mailyan, Z. Gu, D. M. Guptill, H. Wang, H. M. L. Davies, A. Zakarian, J. Am. Chem. Soc. 2014, 136, 17738–17749;
- 7gC. Zheng, I. Dubovyk, K. E. Lazarski, R. J. Thomson, J. Am. Chem. Soc. 2014, 136, 17750–17756;
- 7hA. Cernijenko, R. Risgaard, P. S. Baran, J. Am. Chem. Soc. 2016, 138, 9425–9428;
- 7iJ. Y. Cha, J. T. S. Yeoman, S. E. Reisman, J. Am. Chem. Soc. 2011, 133, 14964–14967;
- 7jB. J. Moritz, D. J. Mack, L. Tong, R. J. Thomson, Angew. Chem. Int. Ed. 2014, 53, 2988–2991; Angew. Chem. 2014, 126, 3032–3035;
- 7kZ. Pan, C. Zheng, H. Wang, Y. Chen, Y. Li, B. Cheng, H. Zhai, Org. Lett. 2014, 16, 216–219.
- 8
- 8aA. Hou, B. Jiang, H. Yang, Q.-S. Zhao, Z. Lin, H.-D. Sun, Acta Bot. Yunnanica 2000, 22, 197–200;
- 8bW.-G. Wang, J. Yang, H.-Y. Wu, L.-M. Kong, J. Su, X.-N. Li, X. Du, R. Zhan, M. Zhou, Y. Li, J.-X. Pu, H.-D. Sun, Tetrahedron 2015, 71, 9161–9171.
- 9For a similar intramolecular 1,4-addition of eriocalyxin B, see:
- 9aY. Zhao, X. M. Niu, L. P. Qian, Z. Y. Liu, Q. S. Zhao, H. D. Sun, Eur. J. Med. Chem. 2007, 42, 494–502;
- 9bJ.-P. Liu, H.-B. Zhang, S.-X. Huang, J.-X. Pu, W.-L. Xiao, X.-P. Zhang, W.-D. Xiao, C. Lei, H.-D. Sun, J. Heterocycl. Chem. 2012, 49, 571–575.
- 10For similar isomerization reactions, see:
- 10aE. Fujita, M. Node, H. Hori, J. Chem. Soc. Perkin Trans. 1 1977, 611–621;
- 10bL. J. Benjamin, G. Adamson, L. N. Mander, Heterocycles 1999, 50, 365–375;
- 10cL. Zhu, Y. Han, G. Du, C.-S. Lee, Org. Lett. 2013, 15, 524–527.
- 11For a recent review on SmI2-mediated coupling reactions, see: M. Szostak, N. J. Fazakerley, D. Parmar, D. J. Procter, Chem. Rev. 2014, 114, 5959–6039.
- 12“Overbred intermediates” refers to intermediates that are structurally more complex than the target, with one or more excess C−C bonds cleaved in subsequent transformations: R. W. Hoffmann, Elements of Synthesis Planning, Springer, Berlin, 2009, pp. 106–117, and references therein.
10.1007/978-3-540-79220-8 Google Scholar
- 13Y. Lu, H. Yuan, S. Zhou, T. Luo, Org. Lett. 2017, 19, 620–623.
- 14For the first synthesis of 11, see:
- 14aD. Liotta, C. Barnum, R. Puleo, G. Zima, C. Bayer, H. S. Kezar, J. Org. Chem. 1981, 46, 2920–2923; for a scalable synthesis of 11, see:
- 14b“Dual-Mode Lewis Acid Induced DA/carbocyclization Cascade Reaction and Its Application to the Synthesis of ent-Kaurene-type Natural Products”: L. Zhu, PhD Dissertation, Peking University, 2014.
- 15S. Arai, K. Nakayama, K.-i. Hatano, T. Shioiri, J. Org. Chem. 1998, 63, 9572–9575.
- 16H.-J. Liu, E. N. C. Browne, S. Y. Chew, Can. J. Chem. 1988, 66, 2345–2347.
- 17CCDC 1581177 (S3), 1581351 (22), 1581233 (26 a), and 1581231 (4 a) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 18
- 18aN. Kornblum, J. W. Powers, G. J. Anderson, W. J. Jones, H. O. Larson, O. Levand, W. M. Weaver, J. Am. Chem. Soc. 1957, 79, 6562;
- 18bN. Kornblum, W. J. Jones, G. J. Anderson, J. Am. Chem. Soc. 1959, 81, 4113–4114;
- 18cB. Ganem, R. K. Boeckman, Jr., Tetrahedron Lett. 1974, 15, 917–920.
10.1016/S0040-4039(01)82368-6 Google Scholar
- 19
- 19aI. Shimizu, F. Aida, Chem. Lett. 1988, 17, 601–604;
- 19bJ. Tsuji, T. Mandai, Synthesis 1996, 1–24.
- 20Epimerization of the C5 stereogenic center for the synthesis of ent-kauranoids has recently been carried out by Ma and co-workers using DBU; see Ref. [3v].
- 21G. M. Rubottom, M. A. Vazquez, D. R. Pelegrina, Tetrahedron Lett. 1974, 15, 4319–4322.