Selective C−H Bond Cleavage in Methane by Small Gold Clusters
Graphical Abstract
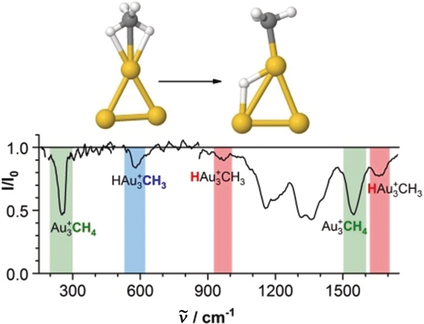
Selection by gold: Vibrational spectroscopy in conjunction with first-principle calculations demonstrate that small gold clusters can mediate the selective dissociation of one of the four C−H bonds of methane, which thus represents the first step towards its conversion into higher-quality chemicals. The distinct selectivity of these gold clusters originates from their finely balanced electronic structure. Au yellow, C gray, H white.
Abstract
Methane represents the major constituent of natural gas. It is primarily used only as a source of energy by means of combustion, but could also serve as an abundant hydrocarbon feedstock for high quality chemicals. One of the major challenges in catalysis research nowadays is therefore the development of materials that selectively cleave one of the four C−H bonds of methane and thus make it amenable for further chemical conversion into valuable compounds. By employing infrared spectroscopy and first-principles calculations it is uncovered herein that the interaction of methane with small gold cluster cations leads to selective C−H bond dissociation and the formation of hydrido methyl complexes, H-Aux+-CH3. The distinctive selectivity offered by these gold clusters originates from a fine interplay between the closed-shell nature of the d states and relativistic effects in gold. Such fine balance in fundamental interactions could prove to be a tunable feature in the rational design of a catalyst.