Traversing Biosynthetic Carbocation Landscapes in the Total Synthesis of Andrastin and Terretonin Meroterpenes
Dr. Gong Xu
Department of Chemistry, University of California, Berkeley, 826 Latimer Hall, Berkeley, CA, 94720 USA
Search for more papers by this authorMasha Elkin
Department of Chemistry, Yale University, 275 Prospect Street, New Haven, CT, 06520 USA
Search for more papers by this authorProf. Dr. Dean J. Tantillo
Department of Chemistry, University of California, Davis, 1 Shields Avenue, Davis, CA, 95616 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Timothy R. Newhouse
Department of Chemistry, Yale University, 275 Prospect Street, New Haven, CT, 06520 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Thomas J. Maimone
Department of Chemistry, University of California, Berkeley, 826 Latimer Hall, Berkeley, CA, 94720 USA
Search for more papers by this authorDr. Gong Xu
Department of Chemistry, University of California, Berkeley, 826 Latimer Hall, Berkeley, CA, 94720 USA
Search for more papers by this authorMasha Elkin
Department of Chemistry, Yale University, 275 Prospect Street, New Haven, CT, 06520 USA
Search for more papers by this authorProf. Dr. Dean J. Tantillo
Department of Chemistry, University of California, Davis, 1 Shields Avenue, Davis, CA, 95616 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Timothy R. Newhouse
Department of Chemistry, Yale University, 275 Prospect Street, New Haven, CT, 06520 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Thomas J. Maimone
Department of Chemistry, University of California, Berkeley, 826 Latimer Hall, Berkeley, CA, 94720 USA
Search for more papers by this authorGraphical Abstract
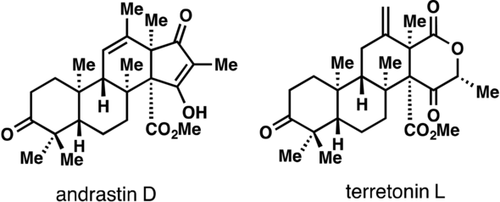
All positives: A key point of divergence in dimethylorsellinic-acid-derived meroterpene biosynthesis is the protoaustinoid A carbocation, which can be diverted to either the berkeleyone, andrastin, or terretonin structural classes by cyclase-controlled rearrangements. Shown herein is that the carbocation can be reverted to either andrastin or terretonin ring systems under abiotic reaction conditions. The first total syntheses of members of these natural product families are reported as their racemates.
Abstract
Meroterpenes derived from dimethylorsellinic acid (DMOA) and farnesyl pyrophosphate have attracted much biosynthetic attention, yet only recently have synthetic solutions to any family members appeared. A key point of divergence in DMOA-derived meroterpene biosynthesis is the protoaustinoid A carbocation, which can be diverted to either the berkeleyone, andrastin, or terretonin structural classes by cyclase-controlled rearrangement pathways. Shown herein is that the protoaustinoid bicyclo[3.3.1]nonane nucleus can be reverted to either andrastin or terretonin ring systems under abiotic reaction conditions. The first total syntheses of members of these natural product families are reported as their racemates.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201705654-sup-0001-misc_information.pdf4.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. Geris, T. J. Simpson, Nat. Prod. Rep. 2009, 26, 1063;
- 1bY. Matsuda, I. Abe, Nat. Prod. Rep. 2016, 33, 26;
- 1cY. Matsuda, T. Awakawa, T. Mori, I. Abe, Curr. Opin. Chem. Biol. 2016, 31, 1.
- 2
- 2aR. Cox, Nat. Prod. Rep. 2014, 31, 1405;
- 2bT. J. Simpson, D. J. Stenzel, J. Chem. Soc. Chem. Commun. 1981, 1042;
- 2cF. E. Scott, T. J. Simpson, L. A. Trimble, J. C. Vederas, J. Chem. Soc. Chem. Commun. 1986, 214;
- 2dY. Matsuda, T. Awakawa, T. Wakimoto, I. Abe, J. Am. Chem. Soc. 2013, 135, 10962;
- 2eH.-C. Lo, R. Entwistle, C.-J. Guo, M. Ahuja, E. Szewczyk, J.-H. Hung, Y.-M. Chiang, B. R. Oakley, C. C. C. Wang, J. Am. Chem. Soc. 2012, 134, 4709;
- 2fY. Matsuda, T. Iwabuchi, T. Fujimoto, T. Awakawa, Y. Nakashima, T. Mori, H. Zhang, F. Hayashi, I. Abe, J. Am. Chem. Soc. 2016, 138, 12671;
- 2gT. Itoh, K. Tokunaga, E. K. Radhakrishnan, I. Fujii, I. Abe, Y. Ebizuka, T. Kushiro, ChemBioChem 2012, 13, 1132;
- 2iY. Matsuda, T. Iwabuchi, T. Wakimoto, T. Awakawa, I. Abe, J. Am. Chem. Soc. 2015, 137, 3393;
- 2jY. Matsuda, T. Awakawa, T. Itoh, T. Wakimoto, T. Kushiro, I. Fujii, Y. Ibizuka, I. Abe, ChemBioChem 2012, 13, 1738;
- 2kY. Matsuda, Z. Quan, T. Mitsuhashi, C. Li, I. Abe, Org. Lett. 2016, 18, 296;
- 2lY. Matsuda, T. Awakawa, I. Abe, Tetrahedron 2013, 69, 8199;
- 2mC.-J. Guo, B. P. Knox, Y.-M. Chiang, H.-C. Lo, J. F. Sanchez, K.-H. Lee, B. R. Oakley, K. S. Bruno, C. C. C. Wang, Org. Lett. 2012, 14, 5684;
- 2nY. Matsuda, T. Wakimoto, T. Mori, T. Awakawa, I. Abe, J. Am. Chem. Soc. 2014, 136, 15326;
- 2oV. Valiante, D. J. Mattern, A. Schüffler, F. Horn, G. Walther, K. Scherlach, L. Petzke, J. Dickhaut, R. Guthke, C. Hertweck, M. Nett, E. Thines, A. A. Brakhage, ACS Chem. Biol. 2017, 12, 1227.
- 3
- 3aC. P. Ting, G. Xu, X. Zeng, T. J. Maimone, J. Am. Chem. Soc. 2016, 138, 14868;
- 3bM. Elkin, S. M. Szewczyk, A. C. Scruse, T. R. Newhouse, J. Am. Chem. Soc. 2017, 139, 1790.
- 4R. Uchida, K. Shiomi, J. Inokoshi, T. Sunazuka, H. Tanaka, Y. Iwai, H. Takayanagi, S. Ōmura, J. Antibiot. 1996, 49, 418.
- 5
- 5aJ. P. Springer, J. W. Dorner, R. J. Cole, R. H. Cox, J. Org. Chem. 1979, 44, 4852;
- 5bG.-Y. Li, B.-G. Li, T. Yang, J.-H. Yin, H.-Y. Qi, G.-Y. Liu, G. L. Zhang, J. Nat. Prod. 2005, 68, 1243;
- 5cX.-H. Liu, F.-P. Miao, M.-F. Qiao, R. H. Cichewicz, N.-Y. Ji, RSC Adv. 2013, 3, 588;
- 5dT. Fukuda, Y. Kurihara, A. Kanamoto, H. Tomoda, J. Antibiot. 2014, 67, 593.
- 6For a complete list of terretonin structures isolated to date, see the Supporting Information.
- 7R. Okamoto, K. Takeda, H. Tokuyama, M. Ihara, M. Toyota, J. Org. Chem. 2013, 78, 93.
- 8For a review of DFT calculations on terpene-forming carbocation rearrangements, see: D. J. Tantillo, Nat. Prod. Rep. 2011, 28, 1035.
- 9Gaussian 09, revision D.01, M. J. Frisch et al., Gaussian, Inc., Wallingford, CT, 2013.
- 10For systematic evaluation and recommendation of using B3LYP geometries and mPW1PW91 single point energies, see: S. P. T. Matsuda, W. K. Wilson, Q. Xiong, Org. Biomol. Chem. 2006, 4, 530.
- 11H. Shigehisa, T. Aoki, S. Yamaguchi, N. Shimizu, K. Hiroya, J. Am. Chem. Soc. 2013, 135, 10306.
- 12For a recent review on metal-mediated radical olefin hydrofunctionalization, see: S. W. M. Crossley, C. Obradors, R. M. Martinez, R. A. Shenvi, Chem. Rev. 2016, 116, 8912.
- 13The corresponding free vinylogous acid of 21 was not compatible with the reaction conditions of this transformation as the N-fluoropyridinium salt led to significant byproduct formation even in the absence of the CoII catalyst.
- 14Demethylation and remethylation of 21 yielded an inseperable ≈1:1 mixture of 21 and its corresponding vinylogous ester regioisomer (iso-21). When this mixture was heated with p-TsOH in PhH we detected small amounts of the ring-shifted product, but only from iso-21. The published synthetic pathway to 20 however, cannot be used to make iso-21 selectively since this regioisomer does not undergo a key bridgehead deprotonation reaction (see Ref. [3a]). Thus this was not considered to be a viable option for the synthesis of andrastins.
- 15J. Justicia, L. Álvarez de Cienfuegos, A. G. Campaña, D. Miguel, V. Jakoby, A. Gansäuer, J. M. Cuerva, Chem. Soc. Rev. 2011, 40, 3525.
- 16For recent examples of 3-exo radical cyclizations initiated by a metal-catalyzed HAT process, see: J. C. Lo, et al., J. Am. Chem. Soc. 2017, 139, 2484.
- 17B. Gaspar, E. M. Carreira, Angew. Chem. Int. Ed. 2008, 47, 5758; Angew. Chem. 2008, 120, 5842.
- 18Similar results are obtained by replacing TsCl with tert-butyl hydroperoxide (TBHP). Presumably these reagents oxidize the CoII precatalyst to CoIII which is required for the intial HAT.
- 19S. W. M. Crossley, F. Barabé, R. A. Shenvi, J. Am. Chem. Soc. 2014, 136, 16788.
- 20DFT studies on a model system with a simplified A ring indicate that the radical rearrangment is both facile and significantly exergonic. The conversion of model radical 24 into 26 is downhill by 8 kcal mol−1 with activaton barriers of 11.6 kcal mol−1 for 24→25 and 4.9 kcal mol−1 for 25→26 (see the Supporting Information).
- 21CCDC 1570755 (9), 1570756 (10), and 1570754 (22) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.