Supercharging Proteins: How Many Charges Can a Protein Carry?
Graphical Abstract
Very highly charged proteins, so-called “supercharged” ions, can lose (excess) protons to background gases like N2. It is remarkable that such extremely acidic species can be generated in electrospray ionization, in the presence of not just N2 but also much higher-basicity solvents. What mechanism(s) can explain such high charging, and what is the ultimate limit?
The 2002 Nobel Prize in Chemistry recognized the importance of the “soft ionization techniques electrospray ionization (ESI)1 and matrix-assisted laser desorption/ionization (MALDI) for mass spectrometric analyses of biological macromolecules”. A major difference between ESI and MALDI lies in the multiple-charging behavior of ESI, as opposed to attachment of a single charge in MALDI. While both protein cations and anions can be generated by ESI, cations are generally much more stable, and expressly multiply-protonated ions can readily be formed. Multiple charging of large molecules, such as proteins, presents some key advantages. It brings the mass-to-charge ratio, m/z, to lower values, where mass spectrometric performance, such as mass resolving power and mass accuracy, are maximized. In detection schemes where the number of charges are detected, higher charging results in lower detection limits, and thus higher sensitivity. Finally, when fragmenting ions, it is advantageous to have a large number of charges on the precursor ions, to minimize undetectable neutral fragments.
Given these advantages, efforts in the past decade and a half have aimed at increasing the charge states of proteins, in so-called “supercharging” experiments.2 This raises the intriguing question what fundamental limitations there are to attaching charges, in this case protons, to proteins. A recent Communication by Donald and co-workers3 presents evidence that highly-charged protein ions are so acidic that they can lose protons to N2, O2, and even He! Given that electrospray is effected at atmospheric pressure, often using N2 drying gas, it seems remarkable then that such highly-charged species can even be formed, and how mechanistically one could explain it. Before we consider the factors that are thought to limit charging, let us briefly review some of the underlying assumptions and hypotheses.
In electrospray ionization,4 a spray of fine droplets is formed in the presence of an electric field. The droplets have a net positive charge when a positive voltage is applied. As solvent evaporates from the droplets, the Coulomb charge repulsion eventually exceeds the surface tension of the droplet, leading to droplet fission. This Coulomb fission process may be repeated many times before minute nanodroplets are formed.
Three mechanisms5, 6 have been put forward to rationalize generation of gaseous ions from nanodroplets (Scheme 1). In the charge residue model (CRM), the nanodroplet contains a single analyte molecule. Evaporation of the remaining solvent molecules leads to bare ions in the gas phase. In the ion evaporation model (IEM), the analyte molecule is evaporated directly from the nanodroplet due to high electric fields at the surface of the droplet. In the recently proposed chain ejection model (CEM), an elongated chain is ejected from the droplet first, followed by sequential ejection of the remaining molecule.
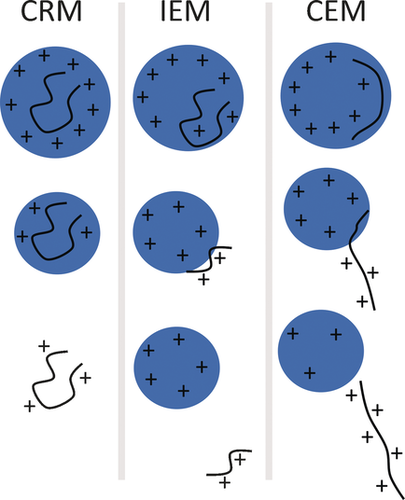
It is possible that all of these postulated mechanisms operate to varying degrees, depending on the molecular systems, and even on the experimental conditions. For instance, small molecules are widely thought to be ionized via the IEM, whereas ionization of large globular macromolecules is more compatible with the CRM. CEM may apply to unfolded, poorly solvated structures.
It has long been known that the degree of folding or unfolding of proteins in solution have an intimate effect on ESI charge state distributions. Whereas folded structures give rise to narrow distributions at low charge states, unfolded structures produce broad distributions at higher charge states. This makes sense, as the charge repulsion in unfolded proteins is minimized, because the distances between the charges are large. Conversely, in folded structures these distances are small, and it is energetically unfavorable to place many charges in close proximity.
In trying to enhance charging, supercharging reagents, such as m-nitrobenzyl alcohol, sulfolane, and 1,2-butylene carbonate, have been found to be particularly effective.2 Like the ESI mechanisms, the underlying mechanism of supercharging continues to be debated. The role of supercharging reagents in destabilizing folded structures, as well as increasing the surface tension of droplets (leading to higher-charged droplets before Coulomb fission occurs), are both compatible with increasing charge states in proteins,2d though direct interactions between the proteins and reagents may also play a role.2b
In the experiments by Donald and co-workers,3 supercharged protein charge states are trapped inside an ion trap in the presence of a controlled gas environment of He, Ar, O2 and/or N2. It is observed that the rate of deprotonation is seemingly related to the gas-phase basicity of the background gas. While deprotonation in He is slow, it is much faster in the atmospheric gases O2 and N2. This strongly suggests that a proton transfer takes place from the protein to the background gas. A more definite proof would be the appearance of protonated N2, O2, etc.; however, such low masses cannot be trapped in their ion trap.
In another insightful experiment, they controlled the gas composition in the ESI source, using the same standard gases. Once again, an N2-enriched ion source environment results in lower charge states compared to a He-enriched environment. This strongly suggests that the background gas in the ion source is a key limiting factor in attaining higher charge states. Right now, about every 4th amino acid residue can be protonated on average, but that may be pushed to every 3rd residue in for instance a lower-pressure environment, in which case the proton would have to “boil off” into vacuum.
Donald and co-workers also approximated the gas-phase basicity, GB, as a function of charge state. GB for molecule M is defined as the free energy of the reaction MH+(g)→M(g)+H+(g). The higher the GB value, the more difficult it is to abstract the proton from the molecule, and thus the more basic the molecule is. Extending on the work by Williams and co-workers,7 the GB values predictably decrease with increasing charge states, as it becomes progressively harder to attach protons on the same molecule. What is surprising, however, is that the GB values for the highest charge states are extremely low, as low as O2 and N2.
Experimentally, the GB of a molecule is established in ion-molecule reactions, where a strong-enough base is able to pull the proton from the ion. However, the measurement of accurate GB values for multiply-charged ions is confounded by the complex energetic picture for proton transfer in such ion–molecule reactions, as shown in Scheme 2.7 Assume two molecules, M and B, with identical GB values. For the proton transfer reaction MH++B→M+BH+, the energy landscape looks pretty much symmetrical around the stabilized proton-bound dimer complex, M⋅⋅⋅H+⋅⋅⋅B. For the MH22+ reaction, however, this symmetry breaks down, as an energetic barrier, Ea, is seen in the MH++BH+ exit channel. This barrier arises due to the Coulombic repulsion energy between MH+ and BH+, which is not present for MH22+ and B. This phenomenon is well described in electron-binding in multiply-charged anions, where the (reverse) repulsive Coulomb barrier can trap excess electrons, and can prevent detachment, even when the detachment energies exceed the electron binding energy.8 For the subsequent MH33++B→MH22++BH+ reaction, the contribution of the Coulombic repulsion increases further, though this is compensated by the fact that the MH22++BH+ products are more favored thermodynamically, as GB(B) ≫ GB(MH22+). In this trade-off, Ea is ultimately reduced in higher protein charge states, as progressively less basic sites are populated, and Coulombic repulsion within the protein becomes appreciable. Nonetheless, the presence of the Coulombic barrier leads to an overestimation of the GBs of multiply-charged ions, as a stronger base is required to abstract a proton from the multiply-charged ion than its inherent GB would suggest.
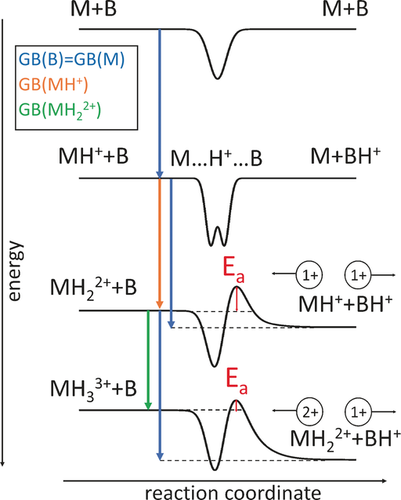
Energy diagrams for proton transfer reactions in ion–molecule reactions as a function of charge state, showing gas-phase basicities (GB), activation barriers (Ea), and complex stabilization.7 Energy effects are much exaggerated for easier depiction. Coulomb repulsion is indicated.
Let us come back to the question how such highly-charged ions can be formed in the first place. Any molecule should be able to steal a proton from these species, and yet they survive. This raises legitimate questions about the ionization mechanism(s). Can the CRM be compatible with such high charge states in the presence of higher-basicity solvents? A related question is how these charge states persist in the high-pressure environment of the ion source, when they readily deprotonate in the lower-pressure ion trap (though granted at much longer residency times). This may suggest that the energetics of the ions in the ion source are significantly different to those in the ion trap. Based on the Coulombic barriers in Scheme 2, one would assume that lower internal energies of the ions in the source could rationalize suppression of proton transfer to N2. Yet, so far, exactly the contrary approach, thermal heating of ESI droplets via high ESI voltages, has been shown to produce supercharging.9
In conclusion, the important contribution by Donald and co-workers, and many others, have raised as many questions, as they have answered, and this will hopefully stimulate more research into the incredibly useful, but also mysterious, technique of electrospray ionization.
Acknowledgements
The author would like to thank NIH (GM110077) and NSF (CHE-1403262) for research support.
Conflict of interest
The author declares no conflict of interest.