Mechanically Interlocked Molecules (MIMs)—Molecular Shuttles, Switches, and Machines (Nobel Lecture)†
Copyright© The Nobel Foundation 2016. We thank the Nobel Foundation, Stockholm, for permission to print this lecture.
Graphical Abstract
Chemistry welcomes a new bond: The mechanical bond has endowed molecules with component parts whose movements can be controlled and monitored. In his Nobel Lecture, J. F. Stoddart describes how being able to template the formation of mechanically interlocked molecules has led to the design and synthesis of shuttles, switches, and machines at the nanoscale.
Preamble
One of the most influential books to have been written in the field of chemistry is The Nature of the Chemical Bond by Linus Pauling,1 the first edition of which was published in 1939. In this classic work, Pauling distinguishes between electrostatic, covalent and metallic bonds, while recognizing that chemical bonds, between two atoms or groups of atoms, exist when the forces acting between them lead to the formation of aggregates we call molecules. Three decades later came the realization that there is another field of chemistry that exists beyond the molecule, which Donald Cram2, 3 referred to as host–guest chemistry, and Jean-Marie Lehn4, 5 as supramolecular chemistry. Chemistry beyond the molecule relates to organized entities that can be neutral molecules, or even cations, anions or radicals, which come together to form higher-order aggregates—call them adducts or complexes—under the influence of stabilizing intermolecular forces that are considerably weaker than are the covalent bonds which define the entities themselves.
The process involving the coming together by organized entities is often referred6 to as self-assembly and the noncovalent bonding that accompanies it as molecular recognition. The noncovalent bonds include hydrogen and halogen bonds amidst a gamut of weak interactions which have been exploited to considerable effect during the past half-century. On this time-scale, another type of bonding in chemistry had been lurking in the background. That bond is the mechanical bond,7 which was the subject of an extensive treatise8 entitled, The Nature of the Mechanical Bond: From Molecules to Machines, published as recently as 2016. Just as the chemical bond is associated in our minds with attractive forces, such as those associated with the sharing of electrons between atoms or the electrostatic forces that exist between ions of opposite charges, the mechanical bond is first and foremost a physical bond which is governed in the final analysis by repulsive forces that prevent chemical bonds from intersecting. Whereas chemical bonds are shared between atoms or groups of atoms, mechanical bonds are shared between molecular entities called component parts. It follows that a mechanical bond can be defined as an entanglement in space between two or more component parts such that they cannot be separated without breaking or distorting the chemical bonds between atoms.
Mechanically Interlocked Molecules
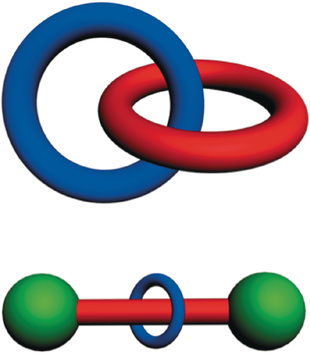
We have referred to molecules that possess mechanical bonds as mechanically interlocked molecules or MIMs for short. The two archetypal examples (Figure 1) of MIMs are the catenanes and the rotaxanes which were the subject of a monograph9 published in 1971 entitled Catenanes, Rotaxanes, and Knots by Gottfried Schill. A catenane, whose name is derived from the Latin word catena, meaning chain, is a molecule with two or more topologically linked macrocyclic component parts, while a rotaxane, whose name is derived from the Latin words rota for wheel and axis for axle, is a molecule comprising at least one macrocyclic component part, i.e., ring(s) with at least one linear component part, i.e., axle(s), threaded through the ring(s) and terminated by bulky end-groups (stoppers) large enough to prevent dethreading of the dumbbell(s) resulting from the existence/formation of chemical (covalent and/or coordinative) bonds between the stoppers and the axle(s). Two points are worthy of mention at this juncture. Catenanes and rotaxanes are molecules: they are not supramolecular entities or supermolecules despite the fact that they most likely will harbor intramolecular noncovalent bonds. While catenanes assume the non-trivial topologies of links, rotaxanes are topologically trivial for the simple reason that their component parts may be separated by continuous deformation, e.g., expanding the diameter of a ring or shrinking the cross-section of a stopper, both of which have been demonstrated8 chemically. Related is the physical process of making rotaxanes known as slippage.10, 11
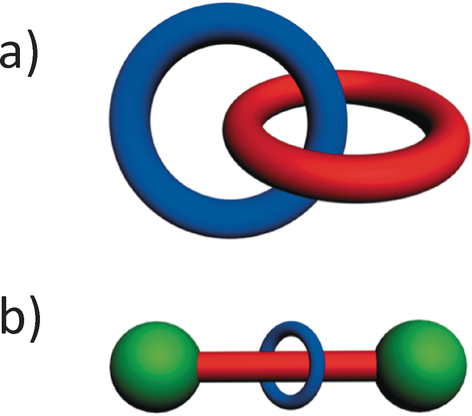
Graphical representations of a) a catenane and b) a rotaxane. A catenane is a mechanically interlocked molecule consisting of two or more interlocked rings. The word catenane is derived from the Latin catena meaning chain. A rotaxane is a mechanically interlocked molecule consisting of a dumbbell component threaded by one or more rings. The word rotaxane is derived from the Latin rota for wheel and axis for axle.
Olympiadane
In the early 1990s the community were somewhat skeptical about the existence and worth of MIMs. Did they really exist and, if they did, how easy were they to make? If they were easy to make, in what context would they become useful? These were not unreasonable questions and they had to be addressed. In the event, it might well take, as of today, a decade or more to start providing answers to the second question. As far as the first question was concerned, it could be addressed rather easily, given the ability of X-ray crystallographer and structural chemist David Williams at Imperial College London to provide a fast turn around on the solid-state structures of catenanes, and occasionally rotaxanes as well. In the case of donor–acceptor catenanes,12 we were intent on demonstrating that higher-order analogues could be produced using a template-directed protocol.13 In this manner, both [3]- and [4]catenanes were synthesized in good yields overall and fully characterized. The envelope was pushed by two postdoctoral researchers, Anatoly Reder and David Amabilino, who, one after the other, took up the formidable challenge of making a [5]catenane (olympiadane) whose constitution was confirmed14 in 1996 by a solid-state structure (Figure 2), courtesy of David Williams. Ju-Young Lee gilded the lily by synthesizing a branched [7]catenane15 in one step from olympiadane. Both the collection of the crystallographic data, which took a couple of weeks, and the solving of the solid-state structure of the branched [7]catenane after a period of five months constituted a tour de force at the time by David Williams. The presence of 20 disordered PF6− counterions plus disordered solvent molecules on top of a crystallographic symmetry highlights the complexity of the task from both an experimental point of view and a computational one. The solid-state structures of these two higher-order catenanes revealed a veritable array of noncovalent bonding interactions in the form of face-to-face [π⋅⋅⋅π] stacking interactions (cf. DNA) and [C−H⋅⋅⋅π] edge-to-face alignments of aromatic rings which are commonplace in proteins rich in aromatic amino acids, along with multiple [C−H⋅⋅⋅O] hydrogen bonds. It is the installing of these intramolecular (ultimately) interactions that aids and abets the templation that makes it possible to synthesize these higher-order donor–acceptor catenanes. The point one learns from all this information is that it is the same weak interactions that are present in naturally occurring compounds that show up time and and time again in exotic unnatural products. At the level of noncovalent bonding interactions, their presence is ubiquitous throughout biology, chemistry and materials science.
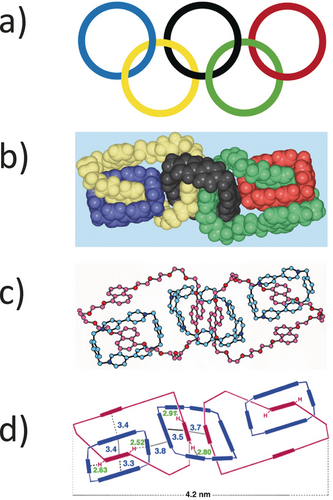
a) The Olympics logo consisting of five interlocked rings picked out from left to right in blue, yellow, black, green and red. b) A space-filling representation of the solid-state structure of a [5]catenane called olympiadane in which the five mechanically interlocked rings are colored according to the Olympics logo. c) A ball-and-stick structure of olympiadane which reveals that two outer blue rings are the same while the blue ring in the middle is a larger homologue of the two terminal rings. The other two red rings enjoy the same constitution in which three 1,5-disubstituted naphthalene rings are linked in a circular fashion, by tetraethylene glycol links. d) A graphical representation of olympiadane where the (red) π-electron rich rings are mechanically interlocked with three (blue) π-electron poor rings.
History
It was an absence of the appreciation by chemists, in the era before there was a realization of the importance of noncovalent bonds and a recognition regime beyond the molecule, that led to a lack of success in the making of catenanes and rotaxanes. Wasserman's synthesis (Figure 3) of a [2]catenane16 in no more than a 1 % yield in 1960 bears witness to the fact that, without any appreciable noncovalent bonding interactions between the precursors to the component parts, the possibility of achieving mechanical interlocking to afford a [2]catenane was largely down to chance, hence the use of the term statistical synthesis to describe much of the research carried out in the 1960s. The achievement of Ed Wasserman (Figure 4) was to serve notice on the chemical community that, although catenation which relies on a chance event is, most likely not going to be efficient, it can be demonstrated. While Wasserman was carrying out his research at Bell Laboratories in Murray Hill, New Jersey, Gottfried Schill and Arthur Lüttringhaus at the University of Freiburg in Germany were devising ways17 by which the component parts of a [2]catenane could be brought together using a covalent bond that could be cleaved in the final steps of the synthesis. The key compound shown at the top in Figure 5 was obtained crystalline after 14 initially linear steps towards the directed synthesis of the [2]catenane shown at the bottom in Figure 5. The authors described the key compound as “one which is linked intra-annularly and in which the chains of the double ansa-system are situated on opposite sides of the benzene ring.” They also drew attention to the fact that the chain in the precursor to the key compound, which is attached by means of a cyclic acetal to the benzene ring, is held at right angles to the plane of the ring on account of the tetrahedral configuration about the aliphatic (acetal) carbon atom, thus ruling out the formation of the isomer with the extra-annular attachment of the ring. The final four steps of the 18-step synthesis all went in nearly quantitative yields to afford the [2]catenane. Aside from it being a long and difficult synthesis, the fact that once all the covalent bonds holding the component parts together have been cleaved, the two mechanically interlocked rings are essentially devoid of any ‘cross-talk’ between them. Nonetheless, Schill continued to be active in the field of directed synthesis of MIMs, including rotaxanes, until the early ’90s. I have commented elsewhere18 that Gottfried Schill (Figure 6) can be looked up as the father of the mechanical bond: he is a chemist who was decades ahead of his time!
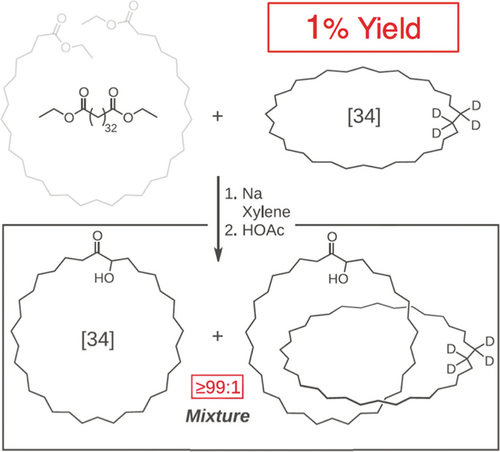
Wasserman's statistical synthesis reported in 1960 of arguably the first wholly synthetic [2]catenane employing an acyloin condensation in order to clip an acyclic diester around a deuterated cyclohexane derivative. The ring sizes, expressed as the number of carbon atoms in the rings, are denoted in square brackets. Deuterium labeling was employed as evidence in support of catenation following cleavage of the α-hydroxyketone functions with alkaline hydrogen peroxide.

Ed Wasserman.
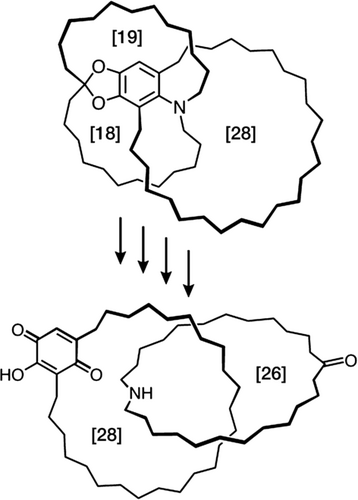
The telescoped final four steps in a 22-step covalent-directed synthesis of a [2]catenane reported by Schill and Lüttringhaus in 1964. The ring sizes, given in terms of the number of carbon atoms present in the rings, are denoted in square brackets.

Gottfried Schill.
A seminal publication19 by Jean-Pierre Sauvage (Figure 7) in 1983 describing (Figure 8) the use of a copper(I) ion to template the formation of catenates—from whence, on demetallation, catenanes can be obtained—was a game-changer. His introduction of transition metal templation into the field of MIMs was transformative: it demonstrated that catenanes and rotaxanes were readily accessible and set the stage for the subsequent emergence and rapid growth of the field.

Jean-Pierre Sauvage.
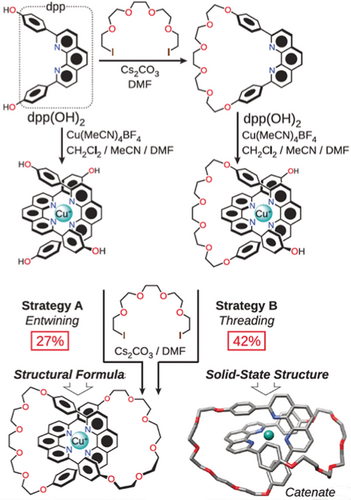
Sauvage's entwining (Strategy A) and threading (Strategy B) for the copper(I)-templated synthesis of the first catenate in 1983. In the bottom right-hand corner is a tubular representation of the solid-state structure of the catenate.
Commercial Building Blocks
During a three-year secondment to the ICI Corporate Laboratory between 1978 and 1981, I joined forces with Howard Colquhoun in the investigation of the second-sphere coordination of transition metal ammines by crown ethers.20 This program of research was sustained in its speed and efficiency by our striking up a highly fruitful collaboration with David Williams. One of the solid-state superstructures he obtained in 1981 was to set us on a road to discovery and invention. The superstructure (Figure 9a,b) in question was that of a 1:1 crystalline adduct that is formed21 when a dicationic platinum ligand in addition to two cis-diammine ligands is crystallized (CH2Cl2/Et2O) in the presence of a molar equivalent of dibenzo[30]crown-10 (DB30C10). While the two ammine ligands from [N−H⋅⋅⋅O] hydrogen bonds with the two polyether loops of DB30C10, the bipyridyl ligand finds itself slotted (Figure 9 c) in between the two catechol rings of the crown ether. The structural similarity between the platinum complex and diquat—a compound marketed by ICI at the time in admixture with paraquat as a ‘wipe-out’ weedkiller—led us to show that it forms22 a 1:1 crystalline complex with DB30C10 as well. Its solid-state superstructure (Figure 9 d,e) mirrors that of the 1:1 adduct DB30C10 forms with the platinum complex. The diquat dication finds itself enjoying (Figure 9 f) charge transfer and π–π stacking interactions with the two catechol rings in the crown ether, aided and abetted by [C−H⋅⋅⋅O] hydrogen bonds.
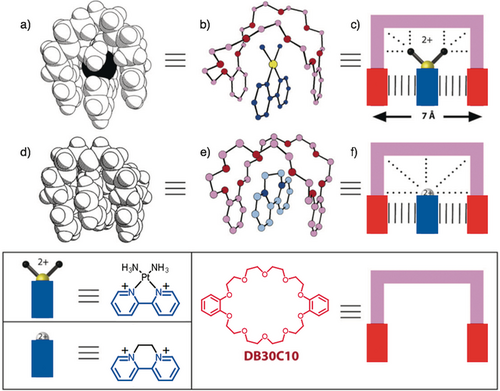
a) Space-filling and b) ball-and-stick representations of the 1:1 adduct formed between [Pt(bipy)(NH3)2]2+ and DB30C10 in the solid state. c) A graphical representation of the 1:1 adduct showing the [π⋅⋅⋅π] stacking interactions (sets of vertical lines) and the [N−H⋅⋅⋅O] hydrogen bonds and pole–dipole interactions between the dicationic transition metal diammine and some of the oxygen atoms in the polyether loops of DB30C10. d) Space-filling and e) ball-and-stick representations of the 1:1 complex formed between the diquat dication and DB30C10. f) A graphical representation showing the [π⋅⋅⋅π] stacking interactions (sets of vertical lines) and the [C−H⋅⋅⋅O] hydrogen bonds between the bismethylene bridge in the diquat cation and some of the oxygen atoms in the polyether loops of DB30C10.
Blue Inside Red and Red Inside Blue
The next step, on my return to Sheffield in 1981, was to uncover a good crown ether receptor for paraquat. We had observed that DB30C10 was able to form a weak 1:1 complex with diquat and so we argued that a small change to the constitution of the crown ether might be all that is required in order to pinpoint a good crown ether receptor for paraquat. This strategy proved to be successful. We found that a constitutional isomer of DB30C10, namely bis-para-phenylene[34]crown-10 (BPP34C10), forms23 a strong 1:1 complex with paraquat. Moreover, the solid-state superstructure (Figure 10 a) of the crystalline 1:1 complex, when compared with the solid-state structure of BPP34C10, carried two very strong messages. One was the fact that the crown ether, at least as portrayed in the solid state, is preorganized to complex with the paraquat (PQT2+) dication. The other was that the manner in which the PQT2+ dication threads through the BPP34C10 ring is highly suggestive of it being a MIM precursor. In the context of molecular recognition, we had established how to bind a π-electron rich host—or using our color scheme, we could put blue inside red. The next question was—could we reverse the recognition motif and put red inside blue? Or expressed another way, could we bind a π-electron rich guest, e.g., 1,4-dimethoxybenzene (1/4DMB), inside a π-electron deficient host? With his synthesis24 of cyclobis(paraquat-p-phenylene) (CBPQT4+), Mark Reddington was able to show that 1/4DMB does indeed bind, albeit somewhat weakly, with CBPQT4+. Once again the solid-state superstructure (Figure 10 b) of the crystalline 1:1 complex, when compared25 with the solid-state structure of CBPQT4+, reveals a highly preorganized match between the guest and the rigid host.
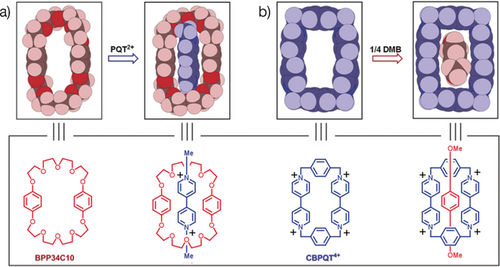
Comparison of the space-filling representations of the solid-state (super)structures of the two receptors a) BPP34C10 and b) CBPQT4+ and their 1:1 complexes obtained, respectively, with paraquat (PQT2+) and 1,4-dimethoxybenzene (1/4DMB). The box at the bottom relates the solid-state (super)structures to the structural formulas for the two receptors, BPP34C10 and CBPQT4+ and their 1:1 complexes with PQT2+ and 1/4DMB, respectively.
First Donor–Acceptor Catenane
We were now poised to perform the all-important experiment which would answer the question—what would happen if we carried out the synthesis of CBPQT4+ in the presence of BPP34C10? Cristina Vicent and Neil Spencer provided the answer by carrying out the reaction (Figure 11) in acetonitrile at room temperature in the presence of BPP34C10 as a template. The outcome from the first reaction exceeded our wildest dreams: we were able to isolate our first donor–acceptor [2]catenane in a remarkable 70 % yield. Our excitement did not stop there: a space-filling representation of the solid-state structure (Figure 12), which graced the front cover of the October 1989 issue of Angewandte Chemie,26 was a sight to behold. Moreover, both 1H NMR spectroscopy and electrochemical experiments, performed at the University of Miami by Angel Kaifer, conveyed a very strong message. The weak noncovalent bonding interactions (Figure 13 a) that are accrued during the template-directed synthesis ‘live on’ in the [2]catenane afterwards. The implications of this observation were to be profound when it came to designing and synthesizing, first of all molecular switches, and then ultimately molecular machines.
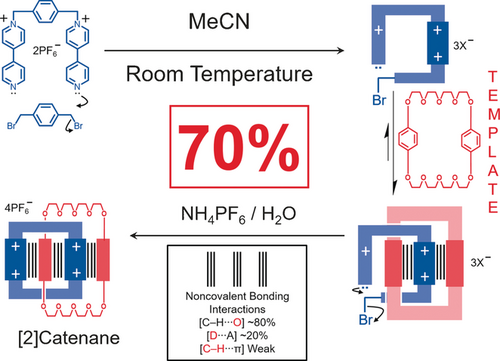
The template-directed synthesis of the first donor–acceptor [2]catenane under kinetic control at room temperature in acetonitrile. BPP34C10, which was present in 3 molar equiv, acts as a template in the stepwise formation of the CBPQT4+ ring in a process where covalent bond formation leads to the production of a bipyridinium unit which is recognized noncovalently by the BPP34C10 when threading occurs: thereafter a second covalent bond is formed resulting in the formation of the [2]catenane where the noncovalent bonding interactions ‘live on’ inside the MIM. They are indicated by the parallel vertical lines.

A space-filling representation of the solid-state structure of the first donor–acceptor [2]catenane adorning of the front cover of the October issue of Angewandte Chemie in 1989.
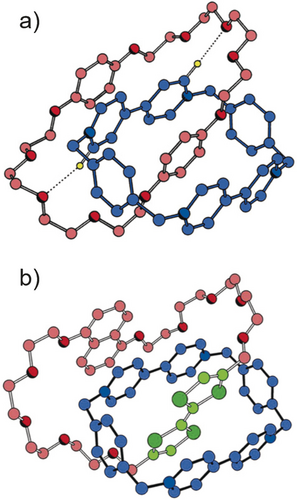
The solid-state structures, depicted in ball-and-state representations, of a) the first donor–acceptor [2]catenane and b) a switchable donor–acceptor [2]catenane based on a stronger tetrathiafulvalene (green) and a weaker 1,5-dioxynaphthalene donor (pink). Note the presence in both [2]catenanes of π–π stacking interactions (3.5 Å) between the aromatic donors and acceptors. In the degenerate [2]catenane, note the presence of strong [C−H⋅⋅⋅O] interactions.
Molecular Shuttle
As we took our leave of Sheffield in 1990 to move to Birmingham, Pier Lucio Anelli had completed the template-directed synthesis (Figure 14) of a degenerate [2]rotaxane27 modeled on that of the degenerate [2]catenane. The lower yield of 32 % reflects the fact that the dumbbell template is much less preorganized than BPP34C10. Dynamic 1H NMR spectroscopy, performed on the rotaxane in hexadeutero acetone, revealed that the CBPQT4+ ring shuttles (Figure 15) back and forth between the two hydroquinone units (‘stations’) around 1000 times a second. For obvious reasons, I found myself referring to the compound as a molecular shuttle and was prompted to draw the following conclusion in the communication27 published with this title in the Journal of the American Chemical Society in 1991:
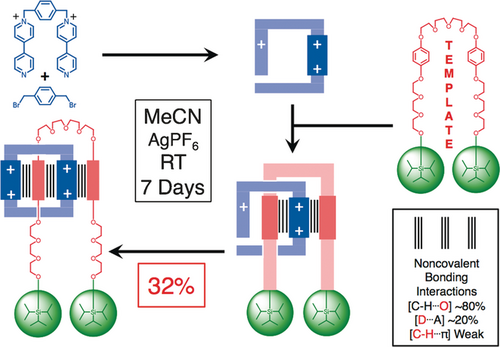
The template-directed synthesis of a degenerate donor–acceptor [2]rotaxane, also known as a ‘molecular shuttle’, under kinetic control at room temperature in acetonitrile. The dumbbell acts as template for the formation of the CBPQT4+ ring in a stepwise manner. The noncovalent bonding interactions which ‘live on’ inside the [2]rotaxane are indicated by parallel vertical lines.
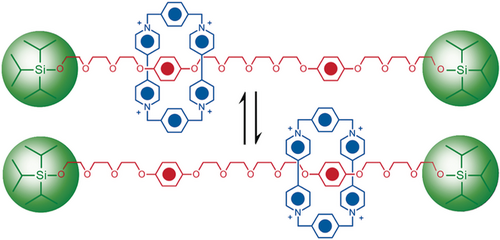
A degenerate donor–acceptor [2]rotaxane for which the phrase ‘molecular shuttle’ was introduced in 1991. The CBPQT4+ ring darts back and forth between the two hydroquinone recognition sites about 1000 times a second in acetone at room temperature. The molecular shuttle was seen as a prototype for the construction of molecular switches and machines based on molecules containing mechanical bonds.
“The opportunity now exists to desymmetrize the molecular shuttle by inserting nonidentical ‘stations’ along the polyether ‘thread’ in such a manner that these different ‘stations’ can be addressed selectively by chemical, electrochemical, or photochemical means and so provide a mechanism to drive the ‘bead’ to and fro between ‘stations’ along the ‘thread’. Insofar as it becomes possible to control the movement of one molecular component with respect to the other in a [2]rotaxane, the technology for building ‘molecular machines’ will emerge.
The molecular shuttle described in this communication is the prototype for the construction of more intricate molecular assemblies where the components will be designed to receive, store, transfer and transmit information in a highly controllable manner, following their spontaneous self-assembly at the supramolecular level. Increasingly, we can look forward to a ‘bottom up’ approach to nanotechnology, which is targeted toward the development of molecular-scale information processing systems.”
Molecular Switches
The next challenge we faced was to desymmetrize the degenerate [2]rotaxane in an attempt to obtain a rotaxane with two recognition units on the dumbbell component, one of which is more attractive to being encircled by the CBPQT4+ ring than the other. After much initial experimentation we settled on a design where one of the hydroquinone units in the molecular shuttle is replaced by a benzidine unit and the other by a biphenol residue. Since we were prevented from using benzidine in the United Kingdom, postdoctoral scholar Richard Bissell made the journey across the Pond in order to complete the template-directed synthesis of the non-degenerate [2]rotaxane in collaboration with Angel Kaifer. On this occasion, dynamic 1H NMR spectroscopy revealed28 that the CBPQT4+ ring spends 84 % of its time on the benzidine unit and 16 % on the biphenol unit at equilibrium at room temperature in CD3CN solution. The unequal distribution of the ring between the two recognition units was sufficient to allow us to intervene in the equilibrium between the two translational isomers. It was possible, by adding acid to protonate the nitrogen atoms on the benzidine unit, to induce (Figure 16), as a result of Coulombic forces, the CBPQT4+ ring to move to the neutral biphenol unit rather than reside on the diprotonated benzidene unit carrying its two positive charges. While this bistable [2]rotaxane was to rank as our first switchable donor–acceptor MIM, a bistable [2]catenane,29 in which one of the two hydroquinone rings in the BPP34C10 component of the original [2]catenane (Figure 13 a) was replaced by a tetrathiafulvalene (TTF) unit and the other with a 1,5-dioxynaphthalene (DNP) unit was designed and synthesized by Gunter Mattersteig. The solid-state structure (Figure 13 b) of this bistable [2]catenane reveals that the TTF unit resides inside the cavity of the CBPQT4+ ring, while the DNP unit is located outside and alongside one of the two bipyridinium (BIPY2+) units in the CBPQT4+ ring.
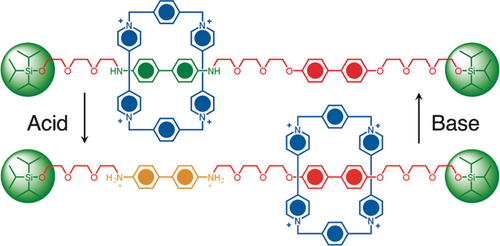
The first switchable donor–acceptor [2]rotaxane, where switching can be implemented by adding a drop of acid to protonate the benzidine recognition unit (the more preferred site for the location of the ring) whereupon the CBPQT4+ ring migrates to the biphenol unit, the less preferred site for the location of the ring on the neutral dumbbell component. The switch can be reset by adding base.
Molecular Electronics
This bistable [2]catenane was used30 to construct a solid-state electronically reconfigurable switch in a simple crossbar device by Jim Heath (Figure 17) when we entered into a highly fruitful collaboration, following my move from Birmingham to the University of California Los Angeles (UCLA). Prior to introducing this bistable [2]catenane into the crossbar device we had ascertained31 how to effect its transfer onto the device using the Langmuir–Blodgett (LB) technique from a Langmuir monolayer where the counterions are dimyristoylphosphatidyl anions. The ON/OFF ratios for the molecular switch tunnel junctions (MSTJs) in crossbar devices incorporating the bistable [2]catenane were only around 2. The desire to increase this ratio took us inexorably in the direction of bistable [2]rotaxanes (Figure 18) which, it transpired, can be switched with an order of magnitude higher ON/OFF ratios. Reversible, electronically driven switching was not only observed in MSTJs incorporating a molecular monolayer of this bistable [2]rotaxane sandwiched between a bottom polysilicon electrode and a top titanium/aluminum electrode, but a 160,000-bit molecular electronic memory circuit has also been fabricated (Figure 19 a) at a density of 100,000,000,000 bits per square centimeter.31,32 The entire 160-kbit crossbar assembly was smaller than the cross-section of a white blood cell and could be switched (Figure 19 b) up to around 100 times. The search for a more robust setting34 in metal–organic frameworks for these bistable MIMs continues.35

Jim Heath.
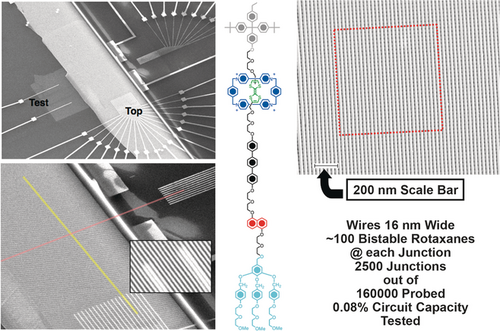
Top left: Scanning electron microscope image of the nanowire crossbar memory. The array of 400 Si bottom nanowires is portrayed as the light grey rectangular patch extending diagonally up from the left. Bottom left: Scanning electron microscope image showing the cross-point of the top (red) and bottom (yellow) nanowire electrodes. Each cross-point corresponds to an ebit in memory testing. Top right: High resolution scanning electron microscope image of approximately 2500 junctions out of a 160,000-junction nanowire crossbar circuit. The red square highlights an area of the memory that is equivalent to the number of bits tested. Positioned in the center of the illustration is the structural formula of the bistable [2]rotaxane used in the memory.

a) A 160-kilobit crossbar molecular memory device (orange in the middle of the illustration) is smaller than the cross-sectional area of a white blood cell (purple). b) A demonstration of point-addressability within the crossbar. Good ebits were selected from the defect mapping of the tested portion of the crossbar. A string of 0s and 1s corresponding to the ASCII characters for ‘CIT’ (California Institute of Technology) were stored and read out sequentially. The dotted line indicates the separation between the 0 and 1 states of the individual ebits. The black trace is raw data showing 10 sequential readings of each bit while the red bars represent the average of these 10 readings.
Drug Delivery Systems
In a collaboration with Jeff Zink (Figure 20) at UCLA, we have functionalized the surfaces of mesoporous silica nanoparticles (MSNPs) with both rotaxane-based nanovalves and snap-tops (Figure 21) for the controlled release of drugs. The nanovalves which adorn the surfaces of the MSNPs can be bistable donor–acceptor [2]rotaxanes36 where redox chemistry can be used to open and close the nanovalves. This type of integrated device holds considerable promise for drug delivery systems.

Jeff Zink.
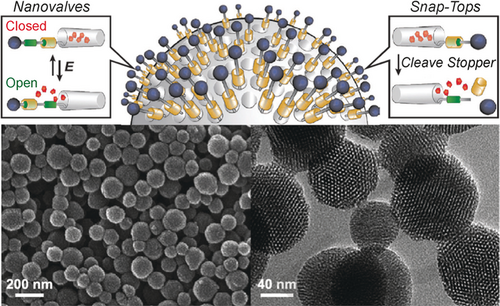
Examples of mechanized mesoporous silica nanoparticles (MSNPs). Illustrations of the mode of operation of rotaxane-based nanovalves and snap-tops for the controlled release of drugs. Scanning electron microscopic (bottom left) image of MSNPs. Transmission electron microscopic (bottom right) image of MSNPs.
Molecular Machines
A fundamental property of biological molecular machines, e.g., the motor proteins,37-44 is that they consume energy and drive systems away from equilibrium by controlling kinetic barriers. The emergence of the science of artificial molecular machines (AMMs) in the hands of chemists, for the most part, presents us with a steep learning curve to negotiate. Nonetheless, it is a challenge that the chemistry community has embraced increasingly during the past two decades with much of the teaching of the fundamental theory coming from the physics community, and, in particular, one very prominent physicist from the University of Maine, namely Dean Astumian45-48 with whom we have collaborated as well as published several reviews49-51 that stand alongside a crop52-69 that can be traced back for at least two decades from the present day. In my Nobel Lecture, I chose to highlight the progress50, 51 we have made in recent times on the design and synthesis of artificial molecular pumps, based on the building blocks that found their origins almost 40 years ago in the ICI Corporate Laboratory and were used by us21-27 to put the mechanical bond on a firm footing before introducing28 them into molecular switches in the first instance.
Unidirectional Transport
At the outset, we designed and synthesized a constitutionally unsymmetrical dumbbell34 and demonstrated (Figure 22) that it transports the CBPQT4+ ring in a unidirectional manner. In its midriff, the dumbbell houses an electron rich 1,5-dioxynaphthalene (DNP) unit which is capable of recognizing the electron poor CBPQT4+ ring. Kinetic control of the threading and dethreading of the dumbbell by the ring is managed by arranging to have a neutral isopropylphenyl (IPP) unit at one end of the dumbbell and a positively charged 3,5-dimethylpyridinium (PY+) unit at the other end. The CBPQT4+ ring finds its most thermodynamically stable location on the DNP unit by passing over the IPP unit for the simple reason that the positively charged ring is repelled by the PY+ unit. When the CBPQT4+ ring is reduced to CBPQT2(⋅ +), the relative heights of the two barriers change on account of the significantly decreased Coulombic barrier confronted by the ring which also, most likely, undergoes a reduction in size, resulting in increasing steric interactions with the PY+ unit. At the same time, the donor–acceptor interactions between the CBPQT2(⋅ +) ring and the DNP recognition unit are nullified, resulting in a preference for the rings to dethread into solution over the charged end of the dumbbell. In what is essentially a supramolecular system, no work is done: rings are extracted out of solution onto the dumbbell momentarily before being released back into solution.
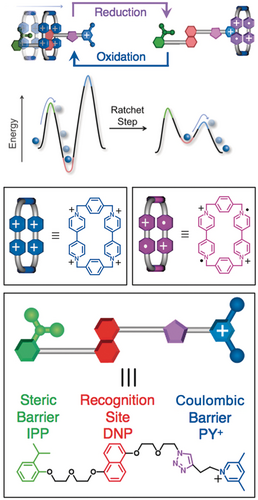
The redox-controlled unidirectional movement of a CBPQT4+ ring along a dumbbell with a neutral steric barrier (green) on the left, a recognition site (red) for the ring under oxidative conditions in the middle and a positively charged Coulombic barrier (blue) on the right. Underneath are energy profiles illustrating the operation of an energy ratchet. The cartoons are defined in the boxes below.
Radical Interactions and Templation
Our initial approach70 to introducing unidirectionality into a supramolecular system employing donor–acceptor interactions demonstrated that we can control the relative motion between a dumbbell and a ring. The system, however, lacks the potential for it to be developed into a more sophisticated molecular machine where useful work can be done since the free energy change during the redox cycle is simply too small to be useful in a practical context. A discovery (Figure 23) made in my research laboratory in 2010 by Ali Trabolsi and Albert Fahrenbach was to come to the rescue. They showed71 that a relatively strong tricationic trisradical complex (Ka=104–105 m−1 in MeCN) is formed between CBPQT2(⋅ +) and bipyridinium (BIPY⋅ +) radical cations under reducing conditions. The strong binding affinity (attraction) can be switched back to being highly repulsive, following oxidation of the radical cations, in both instances, to give CBPQT4+ and BIPY2+, resulting in a massive enthalpy change, something which struck us as being highly promising when it comes to designing and synthesizing AMMs to perform useful work.
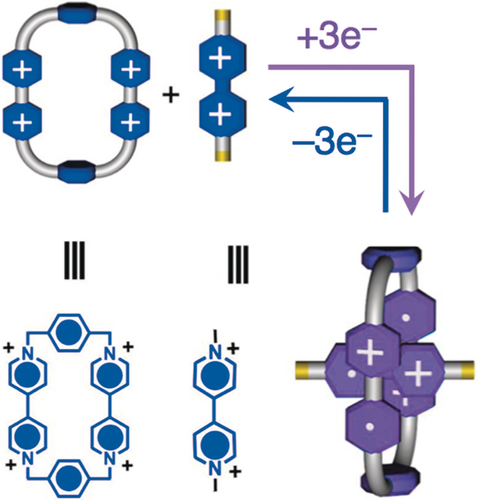
The formation of a CBPQT2(⋅ +) ring and PQT⋅ + on reduction. The dissociation of a CBPQT4+ ring and PQT2+ on oxidation of the 1:1 trisradical tricationic complex.
We reasoned that we could achieve much better and more efficient unidirectional transport of CBPQT4+ rings if we were to replace (Figure 24) the DNP unit in the constitutionally unsymmetrical dumbbell with a BIPY2+ unit and employ radical chemistry to form a stable [2]rotaxane-like tricationic trisradical on reduction of both the ring and the BIPY2+ unit. We had already employed radical templation72 to synthesize (Figure 25 ) a [2]rotaxane—as well as a homo[2]catenane73—and demonstrated (Figure 26) the strong repulsion that comes into play when the ring and recognition unit are oxidized back to CBPQT4+ and BIPY2+, respectively.
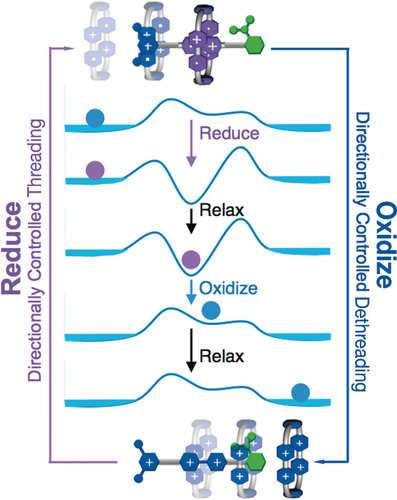
Graphical representations and energy profiles for an artificial molecular pump prototype based on radical–radical stabilizing interactions. In this prototype a bipyridinium radical cation (BIPY⋅ +) centered on a dumbbell with the charged end on the left and the neutral one on the right. The CBPQT2(⋅ +) bisradical dication threads onto the dumbbell over the charged end on the left. On oxidation, a push-button molecular switch comes into action as a result of the generation of six positive charges in place of three that were suppressed previously by radical–radical interactions. The net result is the generation of a lot of potential energy which obliges the CBPQT4+ ring to depart from the neutral right-hand end of the tricationic dumbbell. No work has been done.
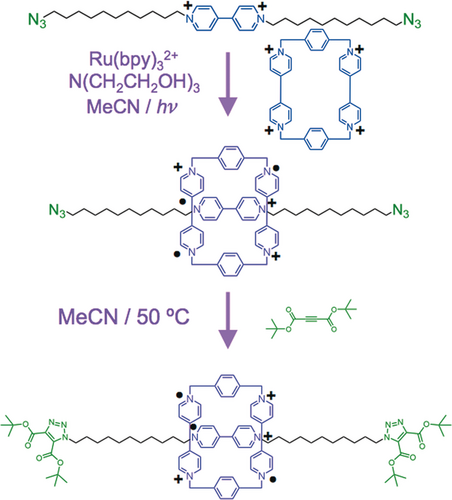
The use of radical templation, promoted by light in the presence of a photosensitizer and sacrificial electron donor, to obtain, following click chemistry, a trisradical tricationic [2]rotaxane.
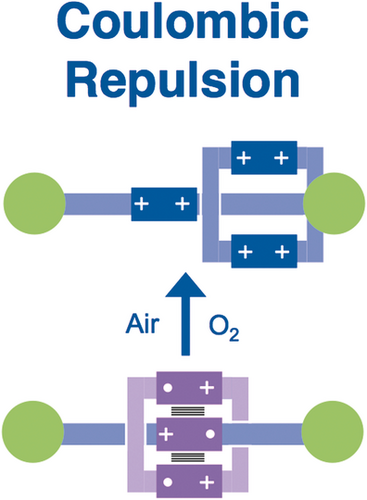
Exposing the trisradical tricationic [2]rotaxane to air produces a hexacationic [2]rotaxane which behaves like a molecular shuttle with an electrostatic barrier to shuttling.
Artificial Molecular Pump
Based on all these previous observations, and a large number of exploratory experiments conducted painstakingly by Chuyang Cheng, he settled on the design (Figure 27) of an artificial molecular pump74, 75—call it Mark I—where an oligomethylene chain terminated by a stopper is attached at its other end to an IPP speed bump so that this portion of the dumbbell can act as a collecting chain for the rings transported from solution. In the oxidized state, the CBPQT2(⋅ +) ring and the molecular pump portion of the dumbbell repel each other. Upon reduction, the CBPQT2(⋅ +) ring passes quickly over the PY+ unit in search of the radical recognition site, namely a BIPY⋅ + unit, to form the thermodynamically favored trisradical tricationic complex. On oxidation, this complex becomes a highly unstable species carrying six positive charges. Under these circumstances, the CBPQT4+ ring would like to relax to a more favored location. Although its returning to the bulk solution is thermodynamically favored, this pathway is blocked kinetically by the PY+ unit, which acts as a Coulombic barrier to deslipping of the ring. Hence, with the aid of thermal energy the ring passes over the IPP speed bump onto the collecting chain. When a second reduction is performed, the IPP unit prevents the association of the trapped ring with the BIPY⋅ + recognition site, enabling this site to attract a second CBPQT2(⋅ +) ring from solution. In this AMM two positively charged rings are collected by a positively charged dumbbell, reflecting a process that is neither entropically nor enthapically favored. Work is done! The artificial molecular pump operates away from equilibrium by relying on the consumption of redox chemical energy. Our findings have shown that the kinetics associated with two cycles are the same to all intents and purposes (Figure 28), suggesting that that the first ring to be trapped does not have a significant influence on the threading of the second ring. These results are promising since they indicate that it is possible to extend the two-cycle system into a multi-cycle one, opening up the possibility of synthesizing slide-ring materials.76 A couple of design modifications to the artificial molecular pump are being explored currently: one is to speed up77 the action of the pump in a Mark II version at least four-fold from two hours per redox cycle to 30 minutes, and the other is to double the addition of rings to polymer chains (Figure 29) by locating pumps at both ends of the chains.
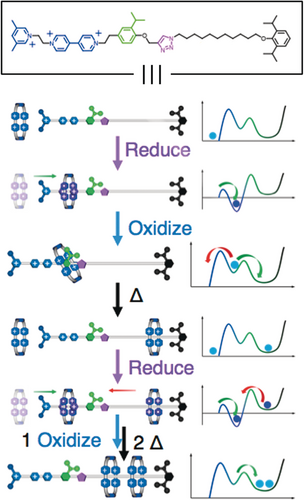
Graphical representations of the pumping mechanism which operates in the case of the artificial molecular pump, portrayed as its structural formula in the box atop the illustration. The energy profiles illustrate two strokes of the pump whereby the CBPQT4+ ring is plucked out of solution in its reduced CBPQT2(⋅ +) form and then forced over a steric barrier (green) onto a long collecting chain by thermal energy following oxidation. Work is done.
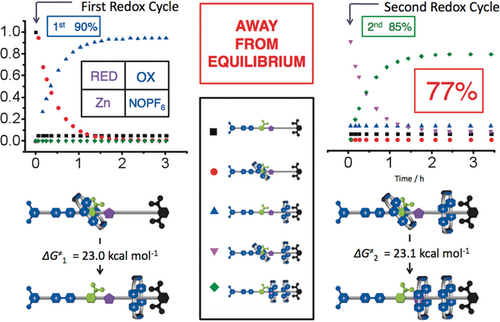
The artificial pump working away from equilibrium. Plots in the change of mole fractions of co-conformations over time in CD3CN at 42 °C during the co-conformational rearrangements that occur after the first cycle to give the [2]rotaxane and after the second cycle to give the [3]rotaxane.
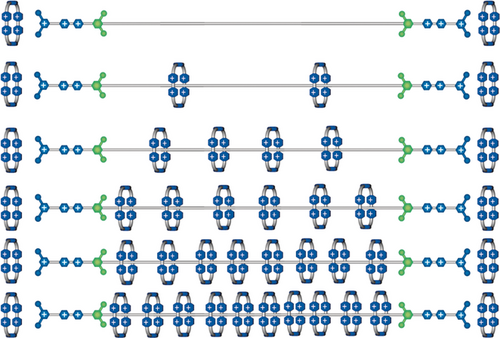
Pumping CBPQT4+ rings in pairs onto polymer chains terminated at both ends with artificial molecular pumps.
Epilogue
In summary, our interest in AMMs was given a considerable fillip in 1991 with the advent27 of the molecular shuttle, and the demonstration28 of the first donor–acceptor bistable switch in 1994. During the past couple of decades, there have been many investigations52, 54, 57 carried out in collaboration with Vincenzo Balzani at the University of Bologna. Our entry into radical chemistry71 and radical templation72 in 2010 set the stage for going forward from entropically driven unidirectional translation with donor–acceptor systems under thermodynamic control in 2013 to enthalpically driven unidirectional transport in 2015 under kinetic control and continuing up to the present time.
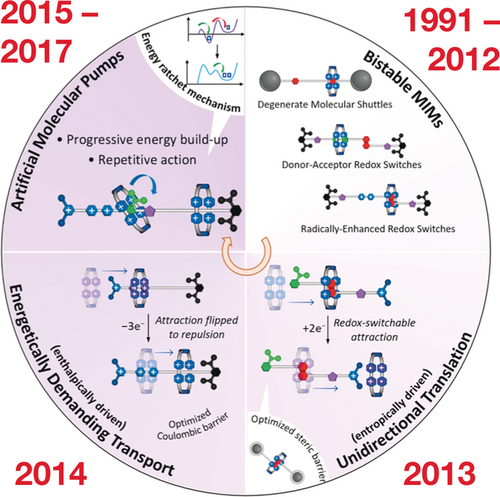
A timeline for the design and synthesis of bistable mechanically interlocked molecules (1991–2012), leading to unidirectional transport (2013) and energetically demanding transport (2014), and artificial molecular pumps (2015–2017).
In a recent Tutorial Review51 in Chemical Society Reviews on “Mastering the Non-Equilibrium Assembly and Operation of Molecular Machines”, written in collaboration with Dean Astumian, we focus on the thermodynamics and kinetics associated with the operation of AMMs and discuss how theory can influence the design principles for constructing molecular machines going forward. A lot of fundamental work remains to be done. It is too early to speak in an authoritative and informed manner about what will be the killer applications of AMMs. We are at the very early stages of knowing how to build them let alone use them. Let me use the analogy of manned flight, particularly in relation to where aviation had reached in 1927, the year in which Charles Lindbergh crossed the Atlantic Ocean in the Spirit of St Louis. An account of the practice of manned flight in 1927 is told78 in all its amazing glory and gory detail in One Summer by Bill Bryson. Compare and contrast the situation for aviation in 1927 with where it stands today as a form of mass transport that has, not only opened up country-wide travel, but also brought Continents together on the grandest of scales. As far as MIMs and AMMs are concerned, they await the engagement of the next generation of chemists eager to exploit the nature of the mechanical bond in chemistry, while taking up the task of designing and synthesizing MIMs and putting them to good use. Figure 31 presents a time-resolved evolutionary tree which highlights the milestones and differentiation events in the synthesis of MIMs. Insofar as the template-directed protocols have established themselves as the most efficient ways to make ‘intelligent’ MIMs, a breakdown8 of the recent literature, based on a random selection of 500 articles on catenanes and rotaxanes, has led to the visualization of the data in a pie chart (Figure 32). Presently, solvophobic, hydrogen bonding, donor–acceptor, and metal templation account for a good three-quarters of the chemical literature on the mechanical bond. The histogram illustrated in Figure 33 a tracks the number of publications on catenanes (red) and rotaxanes (blue), and those containing both MIMs (purple), on a year-by-year basis. Publications relating to the mechanical bond originate (Figure 33 b) from all over the world with the United States, Japan, and the United Kingdom at the top of the list and China not all that far behind. There is every reason to believe that the rapidly accelerating, widespread interest in the chemistry of the mechanical bond, which only started to grow in a linear fashion year-by-year in 1990, is all set now to experience an era of exponential growth.
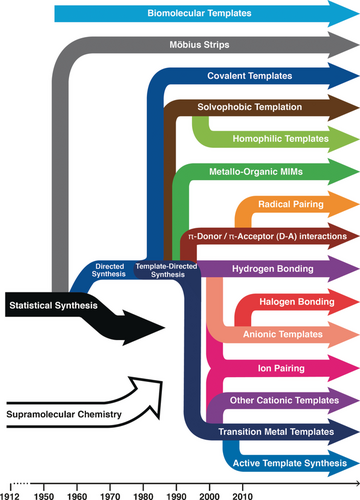
The evolution of the mechanical bond. A time-resolved evolutionary tree highlighting milestones and differentiation events in the template-directed synthesis of mechanical bonds. See Ref. 8.
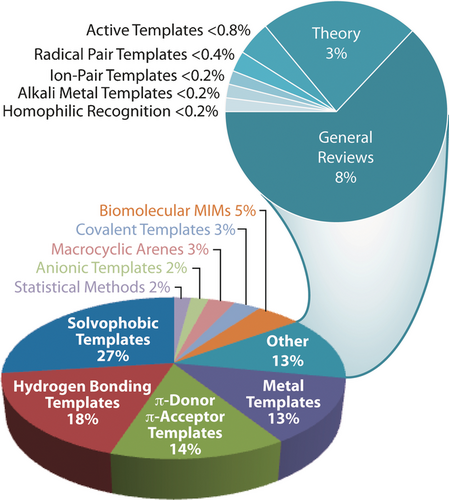
Breakdown of the literature on mechanical bonds according to the mechanisms of their formation/templation. See Ref. 8.

The statistics on journal publications indexed under the keywords, catenanes and rotaxanes. a) The histogram indicates the approximate number of publications (total >3000) from 1960 to 2015. Purple relates to articles indexed under both catenanes and rotaxanes. b) The distribution of publications, indexed under catenane or rotaxane as keywords, color-scaled in green on a map of the world according to their country of origin. See Ref. 8.
Acknowledgements
I wish to put on record my most sincere thanks to the over 400 students, from close on 50 different countries during the past 45 years, who have contributed quite magnificently to the intellectual life as well as the research achievements and impressive productivity coming of laboratories in four universities and one international company, situated in two different countries, namely the United States and the United Kingdom. I acknowledge the constant stimulation and invaluable support in research provided by more than 60 collaborators, some of them colleagues in the five institutions in which I have carried out research and others drawn from research centers of one sort or another, dotted all around the world. I thank all those universities, foundations, research councils, funding agencies and industries who have supported my research enthusiastically and generously—in particular, the Universities of Sheffield and Birmingham in the United Kingdom and at the University of California, Los Angeles (UCLA) and Northwestern University in the United States, Imperial Chemical Industries, the Engineering and Physical Sciences Research Council (and its predecessors), the Biotechnology and Biological Sciences Research Council (and its predecessors) in the United Kingdom, the California NanoSystems Institute, the International Institute for Nanotechnology, the National Science Foundation, the National Institutes of Health, the American Chemical Society, the Department of Energy and the Department of Defense in the United States, and finally, but by no means least, the King Abdulaziz City for Science and Technology in the Kingdom of Saudi Arabia.
Autobiography
I was born in the capital of Scotland on Victoria Day in the middle of World War II. The nursing home in Edinburgh, where this cliff-hanger of an event took place, during the early evening of 24th May 1942, was located at 57 Manor Place. It was not anticipated that a little boy weighing in at just under two kilograms would live until the next morning. I have the doctor's bill, dated 7th October 1942, confirming that I defied the odds he gave against my survival. The bill reads, “Dr. Douglas Miller presents his compliments to Mrs. Stoddart and begs to intimate that his fees for professional attendance amount to £ 4 : 4/-”. It is not the realization that my parents were obliged to pay the princely sum of Four Guineas—equivalent to my father's monthly salary at the time—to have me brought into this world that resonates with me most of all; rather it is the four and a half months the doctor was prepared to wait before sending out his bill to my mother. How times have changed.
My mother, christened Jane Spalding Hislop Fortune, but known as Jean to the family, had made her own way into the world on 23rd May, 1911 at Seggarsdean Farm, in the vicinity of Haddington, a small town about 20 miles east of Edinburgh, in East Lothian. One claim to fame for this town is that it is the birthplace of John Knox, the Scottish minister who was the leader of the Reformation in Scotland and the founder of the Presbyterian Church of Scotland. While still a toddler, Jean Fortune made the move with her parents and two elder brothers, Jim and Tom, to Colstoun Mains which is located three miles south of Haddington. This farm on prime agricultural land was to become the seat of the Fortunes for most of the 20th century. From all reports my mother was quite a sickly child and did not achieve as much as she might have done at school, leaving the Knox Academy in Haddington when she was 14. Her health improved during her teenage years on the farm and eventually she attended the Edinburgh College of Domestic Science on Atholl Crescent, graduating in February 1935 with a First Class Institutional Management Diploma. Following brief experiences as the manageress of private boarding schools in Yorkshire and Devonshire, she became, with financial support from her father, the proud owner and proprietor of the Edenholm Private Hotel in Dunbar, a seaside resort on the North Sea, some eight miles east of Haddington. Reference to old photographs indicate that it was around this time in 1937 that my mother and father met, became engaged and were married in St Cuthbert's Church in Edinburgh on 16th October 1938, just before the onset of World War II in the September of 1939. My maternal grandmother rented a holiday home in Dunbar every summer in the late 1940s so that she could bring all her grandchildren under one roof for a few weeks. I have vivid memories, while in the company of my cousins, watching pigs swim in a paddock on the outskirts of the town during the Great Floods of 1947 that hit the United Kingdom. These holidays by the seaside bring back happy memories, aside from when my grandmother found the urge to have us all visit the unheated swimming pool, open to the chill waters of the North Sea. How we all dreaded the experience that she informed us was good for our constitutions yet apparently not hers!
My father, Thomas Fraser Stoddart, always referred to as Tom by the rest of the family, was born on 20th January 1910 in Irvine, Ayrshire on the West Coast of Scotland. His father was a golf professional who, together with his wife, ran the Bogside Golf Course until he retired in 1945. As summer is the high season for golf, Tom Stoddart, together with his two younger sisters Anna and Clem (short for Clementine), were packed off each summer to the farms of cousins in East Lothian. It was at Howden that my father struck up a close, life-long relationship with his cousin, Tom Scott, while also being bitten by the farming bug. After attending Irvine Academy, my father continued his education at the West of Scotland Agricultural College in Glasgow where, after three years’ training, he gained First Class Certificates in most of his classes and won the McAlpine Memorial Prize as the best student of his year in agricultural botany. I can vouch for the fact that my father knew all that there was to know about grasses to be found in the Lowlands of Scotland. When he graduated from the college in December 1932, the then Principal and Professor of Agriculture was to comment in a testimonial that “He is a young man of energetic and painstaking habits, is methodical in his work, and is possessed of more than the average endowment in grit and determination. These attributes, combined with his sound theoretical and practical knowledge, mark him out as one well fitted for a responsible position in agriculture and dairying.” That position turned out to be the manager of the University of Edinburgh's farms, one of them being Shothead, in the neighborhood of Balerno on the west side of the city, within sight of the Pentland Hills.
Edgelaw
When I was only six months old, my father decided to forsake the comparative comfort and relative security of being a farm manager to take on the tenancy of Edgelaw Farm about a dozen miles south of Edinburgh. Part of the Rosebury Estate, it was the middle of three farms on a dead-end road, which defined its remoteness and lack of electricity until I was almost 18. These circumstances, coupled with the fact that I was an only child, were to define much of my early life's experiences in what I was later to refer to as the ‘University of Life’.

Seated between my father, Tom, and my mother, Jean c. 1946.
I grew up during the 1940s and 1950s in a post-World War II society coping with the rationing of food, clothes, and petrol (gas), and without access to modern-day conveniences in the home and workplace that we take for granted these days. The consequences for me were that I had to live out a very simple lifestyle, and I also had to find ways of amusing myself in a home where only a few rooms through the winter months were habitable. The need for warmth meant that we often lived as a small and close-knit family huddled together in the kitchen, which was fired by a Rayburn cooker that not only provided localized heat, but also hot water for the scullery, wash-house and single bathroom, in addition to some limited cooking space. It was augmented by another gas cooker that was fueled from a large cylinder of rural (liquid) gas. Other rooms in the farmhouse had to be heated by open coal- and wood-burning fires that were often influenced in an unpredictable manner by the wind and rain outside. Up would go the cry that the fire in the drawing room was ‘smoking’, which meant that the room was filling up rapidly with smoke and would soon have to be evacuated. The one and only telephone was located in the hall, which was rarely, if ever, warm, and so conversations tended to be short during the winter months. Light through the long, dark winter months was provided by a vast array of Tilley and oil lamps.
I remember, as if it was only yesterday, the day ‘the electricity,’ as it was called, came to the farmhouse for the first time. It was Christmas Eve 1959 when we received word that the meter man would not be coming to install the meter until the New Year. The disappointment in the household was palpable. We had been so looking forward to celebrating Christmas and the New Year and the long-awaited (17 years!) arrival of ‘the electricity’ with family and friends. I had helped the electrician—a character if ever there was one by the name of Phil MacKay—during the preceding months wire the farmhouse, steading and cottages, and so was pretty knowledgeable when it came to wiring. Unbeknown to my parents, but egged on by Phil, I waited until the cows had been milked and the assembled company were all getting ready to sit down and have a Christmas Eve supper. At that point I fetched a pair of stepladders, climbed up to a point near the ceiling where the meter would eventually be installed, and joined up the first pair of wires between the house and the grid with a pair of pliers. As I expected, nothing of significance happened. On bringing the second pair of wires together, however, there was blinding flash and much of the house was ablaze with light for the first time. There was a lot of noise. My mother was beside herself. She was convinced that someone would report us to the police and we would all end up in jail! Reason prevailed. My mother was soon convinced that by closing the curtains (drapes) in all the rooms we could harbor our secret and have ‘the electricity’ after all. And so it was that for more than a week we had ‘the electricity’ for free and we used it to full advantage. In later years we became much more conscious of switching off lights, for that practice had some bearing on the size on ‘the electricity’ bill. I reckon we all read more and I had no excuse not to do my homework. A television set arrived not so long afterwards and life was never quite the same ever again.
The whole episode brought out the daredevil side of my character. I discovered on the farm that defying regulations and breaking rules was a way to achieve distant goals on a shorter time-scale and, while there might be a price to pay, there would always be supporters, even secret admirers, and after the deed had been done there was no going back.
From a young age, I was addicted to solving jigsaw puzzles and would stack them up when completed between sheets of newspaper. I ascribe my early fascination in stereochemistry and topology to this addiction, which was to give way gradually to one of the more sophisticated of toys in Britain in the 1950s, namely ‘Meccano’. The opportunity to construct a gadget I had designed myself and then put it to work after a fashion was to find expression later on when my passion for the chemical synthesis of unnatural products began to develop. There is also little doubt that my ‘Meccano’ set whetted my appetite many years later for constructing artificial molecular machinery from the bottom up. My interest in tinkering with machines and motors was increased considerably during those times on the farm, when I would take car and tractor engines apart, decoke them, replace the spark plugs and put them back together again, with the prospect that I would be going through exactly the same routine a few months later. The early internal combustion engines were not all that efficient or reliable: they demanded a lot of care and attention.
The late 1940s through the 1950s into the mid 1960s were times of rapid development in agriculture. My parents had no choice but to embrace change like there was no tomorrow. The horse and cart gave way rapidly to the tractor and trailer. The binder and all the labor-intensive and time-consuming paraphernalia that followed in its wake yielded more gradually to the combine harvester and the baler. Our 32 cows, distributed between three byers, were some of the first in the district to be milked by machine. Not all change was seen to be desirable: right up to the last days of the farm in 1968, my mother remained a strong advocate of producing eggs from free-range hens. With the onset of mechanization, collaboration between farmers was commonplace. For all the 26 years that my father ran a flock of 160 lambing ewes, the sheep-shearing was completed in one day (weather permitting) by the shepherds from Colstoun Mains, who would arrive in the early morning with all their motorized clippers. It was an occasion when my mother captured their hearts and souls with a wholesome dinner in the middle of the day that was surely second to none.

Top: Edgelaw Farm House c. 1963. Middle: Kneeling in the middle of the middle row with 11 of my Melville College classmates on my 11th birthday. Bottom: Tethered to a young Ayrshire bull c. 1959.
The farm had two cottages—one for the byerman and the other for the ploughman as well as a bothy (a single-room cottage) that was home to an Irish laborer for part of the year. The cottages experienced a fairly regular turnover of families usually with quite a number of children who were my playmates. We were left free to run wild around the farm and also to roam the countryside at will on our homemade buggies and old bicycles. Creativity and risk-taking came into our play on the grandest of scales in a playground we fashioned to changing circumstances. We invented our own games and learnt the hard way about the dangers of climbing on roofs, burrowing through passages between bales of hay in the hay shed, and speeding down hillsides on carts adorned with a variety of wheels in the summer and on homemade sledges in the winter. The concept of playdates had still to be invented.
My formal education began when I was four years old with mornings only attendance at the local village school in Carrington, around three miles from the farm. My mother recalled that when she collected me from the school at noon on the first day and enquired as to how I had got on, my answer was to ask if I could go the next day for the whole day. At first there were only four other children, all girls, including one, Muriel Logan, a very bright girl from Aikendean Farm. As a consequence of the gender imbalance, I learned to knit, particularly stockings, rather well. By the time I Ieft the village school in 1950, the number of pupils had risen sharply to 28. In this rapidly changing educational environment, where the older pupils helped to look after the younger ones, I discovered very quickly that Miss Morrison did not hesitate to use the tawse—a leather strap having one end cut into thongs that was used by schoolteachers in Scotland in the 1950s as an instrument of punishment—for poor performance, let alone bad behavior.
At the age of eight, my mother decided that I should go to one of the many fee-paying boys’ day schools in Edinburgh. She chose Melville College—formerly the Edinburgh Institution and now, as a result of a merger, Stewart's Melville College—because she was attracted to its predominantly red and black uniform. I was obliged to take an entrance examination which, apparently, I passed with flying colors as a consequence of all that I had learnt in the village school from Miss Morrison. I was blessed with some really outstanding primary school teachers—Miss Christie and Miss Pratt come to mind, both of them now in their nineties and still going strong today. They recall a very shy little boy, shyness being a trait that was to take me more than three decades to overcome.
Before I reached the age of 16, when I could negotiate the journey to school on a Lambretta scooter, my mother would drive me in the family's 1938 Hillman Minx—purchased from James Ross and Sons for £ 155—the three miles to the nearest bus terminal in Rosewell, at that time a small coal-mining village, to catch the 7:40 am bus to Edinburgh. The popularity of cigarette smoking amongst the office workers and shop assistants meant that you could cut the atmosphere with a knife on the top deck of the bus towards the end of the 45-minute journey to St Andrew's Square. The bus journey was followed by a mile-long walk down George Street to the school on Melville Street. I was to realize many years later that the education I received at Melville was second to none, maybe because the 1950s were less than two centuries removed from the period of the Scottish Enlightenment that was graced by eminent scholars, such as philosopher David Hume, economist Adam Smith, poet Robert Burns and chemist Joseph Black. I was taught by teachers, most of whom could have been university professors, in Latin, English Language, English Literature, French, History, Geography, Mathematics, Physics and Chemistry. The school's music master, W. O. (Bill) Minay, was the organist at St Cuthbert's Church—where my parents were married on 19th October 1938—at the West End of Princess Street. It was from Bill Minay that I took piano lessons for many years. With a huge amount of practice and no little encouragement from him, I was able to play the first two movements of Beethoven's First Piano Concerto. Although enjoyable, that experience told me I was not cut out to be a concert pianist.
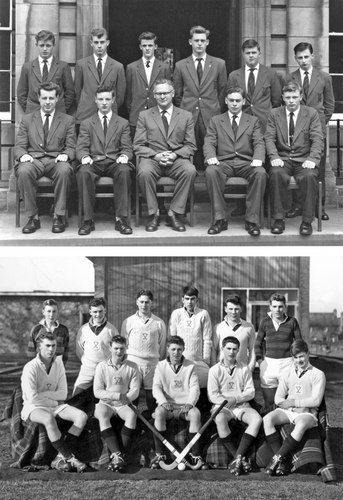
Top: Seated to the right of Mr Richardson, the Headmaster with the other nine school prefects in 1960. Bottom: Third from the left in the back row of the 1960 First Hockey Eleven.
Sport was also a major part of the school curriculum. Rugby, cricket and field hockey were compulsory, along with swimming all the year round. During our last three years in school we found ourselves in army-style uniforms as part of a Cadet Force, in which I rose to become the Signals Sergeant. In my final year, the Headmaster appointed me to be the Second Prefect of the School, a position that gave me adequate opportunities to develop leadership skills. This experience was to prove invaluable when I became Head of the School of Chemistry at Birmingham—and later, the Director of the California NanoSystems Institute.
My mother was a terrific cook and an awesome baker, knowing instinctively when to add and mix ingredients, and rarely, if ever, measuring or weighing anything out. She knew just the right moment to stop whisking, heating and beating mixtures. She went about all these activities and more, including dress-making and patching up clothes, while feeding hens, mucking out henhouses, rearing chickens, gathering eggs and selling them in the neighboring villages and townships. My early successes at practical work in chemical laboratories owed much to watching this remarkable time and motion machine in action. My father, by far the best educated and most well-read farmer in the district, set very high standards for himself, expressed most intensely when a heifer was being groomed to perfection, prior to being sold at the Lanark Stock Market, or a flock of lambs, suitably washed in the dipper and individually manicured to perfection, were on their way to the auctioneer at the St Boswells Sheep Sales.
I was to witness, from a very young age, the essential mating activities that were an integral part of maintaining a herd of dairy cows and orchestrating in October and November the running of around 150 ewes with tups (rams), at an approximate ratio of 50:1 ratio to ensure the arrival of around 250 lambs during a frantic three-week window in March. While tending to cows calving throughout the year on a fortnightly basis, often in the middle of the night, was more or less routine, the lambing season never failed to reduce myself and my parents to states of utter physical and mental exhaustion, from a combination of lack of sleep and very long working days, for spring was also the time to be in the fields from morning to night sowing wheat, barley and oats, to be followed immediately thereafter by potato planting and the sowing of kale and turnips (swedes). In the summer months, I enjoyed nothing more than walking round the 365-acre (one for every day of the year) farm with my father in the evenings of long light. He knew all there was to know about the flora and fauna of the countryside. He was also a walking dictionary—a kind of Google before its time—that was useful for me in building up a vocabulary, and when we were engaged in the evenings in solving crossword puzzles in The Scotsman. My vocabulary was also broadened through my friendship with the farmhands, who taught me to swear from a young age. Later in life, my mother reflected that, much to her chagrin, I could swear like a trooper well before I could talk.
Edinburgh
During my four years as an undergraduate student at Edinburgh (1960–1964), I managed to hold my own in Mathematics, Physics, Chemistry and Biochemistry classes, in the face of stiff competition from many very bright students drawn, in large part, from the east coast of Scotland, many coming from the elite Edinburgh schools. A cohort of English students—who entered the Scottish higher educational system having covered much of the first-year science curriculum at A-level in England—got off to a flying start in their first year, but in subsequent years we Scots started to pull ahead of most of them. The chemistry teaching at Edinburgh in the early 1960s was not particularly taxing or stimulating, apart from some excellent lectures given by Tom Cottrell, John Knox, Peter Schwartz and Dai Rees. Organic chemistry, under the leadership of Professor Sir Edmund Hirst, was heavily skewed towards carbohydrate chemistry. A transformation occurred in my third year during a laboratory course in quantitative analytical chemistry. During his introduction, the somewhat abrasive Dougie Anderson announced to more than 100 of us that we would be pipetting by mouth enough cyanide to kill the whole of Edinburgh! After having made this spine-chilling remark, he went on to state that he had been running the 10-week course for more than a decade and in that time no student had ever completed it. Here was my opportunity, I thought, to apply the multitasking skills I had acquired from working on a mixed-arable farm for a couple of decades. I used this experience and finished the course inside seven weeks, gaining a mark close to 100 %. This achievement earned me my first visit to the office of Sir Edmund who told me that Dr. Anderson would like to offer me a paid position in his research group during the following summer. I jumped at the opportunity. I felt much more at home in this new research environment, where I was given the opportunity to unravel the structural complexities of plant gums of the Acacia genus. There was little doubt from what was already published in the literature that these acidic polysaccharides—accompanied mysteriously by a small amount of protein—were high molecular weight polyelectrolytes constituted around a branched carbohydrate backbone. I was to continue researching these biomacromolecules well beyond a fourth-year research project into the pursuit of a PhD degree as a postgraduate student. My main contribution to the field was to challenge the ‘main-chain’ hypothesis, implying a brush-polymer constitution, and replacing it with a much more highly branched constitution without having the foresight to describe it as a dendrimer before its time. My postgraduate research was to leave me with one lasting impression—namely, that the many gum trees in the Sudan, from whence the nodules I studied came, had never managed to produce between all of them through all of time, two gum molecules which were identical in size and constitution. After this period of handling highly heterogeneous mixtures, I longed to grow acquainted with a molecular world where homogeneity ruled the roost, at least for a time.
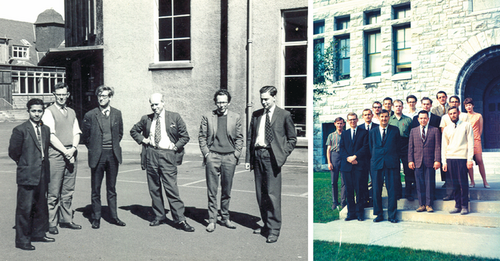
Left: Standing on the right with Douglas Anderson and my fellow postgraduate students at Kings Buildings, University of Edinburgh c. 1965. Right: Second on the right in the third row back with Walter Szarek and Ken Jones at the front taken at Queen's University in Canada c. 1968.
Between continuing to work on the farm, and becoming bitten by the research bug, I had to settle for graduating with a BSc Honours Degree in Chemistry and being the top Upper Second, in fifth place overall, in the 1964 Class of 45 students. By contrast, my postgraduate research was a resounding success and I was able to graduate with a PhD degree in just over two years in November 1966, having met the love of my life, Norma Scholan, who had joined the Anderson group as a fourth year undergraduate research student. Norma made up for my lacklustre performance in my Finals by coming top of her class of over 80 Final Year Chemistry students in 1966. In the years to come our two daughters, Fiona and Alison, were to graduate in Chemistry—from Imperial College London and the University of Cambridge, respectively—with First Class Honours degrees just like their mother before them, leaving me the dunce of the family!
Kingston
During the first 25 years of my life I had travelled very little and I yearned to go to North America, with enthusiastic support from my parents and somewhat less so from Norma, who had transferred her allegiance to the Biochemistry Department in the Medical School to begin her postgraduate work in steroid biosynthesis, under the tutelage of George Boyd. For my part, Sir Edmund sprung into action and did not take long to arrange for me to go to Queen's University in Kingston, Ontario as a National Research Council of Canada Research Fellow. Here I would join the Chemistry group, headed up by Ken Jones, one of his own postgraduate students from his Bristol days. The 1960s witnessed the end of an era in UK chemistry departments, arranging for the department's best students to go overseas to pursue postdoctoral fellowships in research. How times have changed for the better.
I left Prestwick for Montreal aboard a British Overseas Airways Corporation (BOAC) plane, taking to the air for the first time in my life, on 1st March 1967, with Sir Edmund's words ringing in my ears, “Whatever you do in research, Stoddart, make sure you work on a big problem.” I was not at all sure what he meant by a ‘big problem’ but I was determined to heed his advice to the best of my limited ability. I suspect he anticipated that I would remain a carbohydrate chemist for the remainder of my professional life but that did not turn out to be the case. In the event, as soon as I set foot in the Jones’ laboratory, Ken confided in me that come 1st April he would be leaving for Curitiba in Brazil to spend one whole year there on sabbatical leave. This totally unexpected piece of breaking news, although quite a shock for me at the time, was to work to my advantage in the long run. I found myself assisting Walter Szarek, a former graduate student in Ken's group, who had returned to Queen's from Rutgers University to take over its supervision. It was good early experience for me in helping Walter run and mentor a medium-sized research group.
Communications between Canada and Brazil were dependent on the back and forth delivery of airmail letters, with a complete turn around of information taking about three weeks, by which time the news was often obsolete. We were quickly relieved of this frustration when the Canadian postal service was brought to a halt by strikes for months on end. These circumstances left me with enough time on my hands to go in search of Sir Edmund's ‘big problem’. I stumbled upon it in the chemistry department library under the guise of a short communication by Charles Pedersen in the Journal of the American Society (JACS) in the Spring of 1967, describing the efficient template-directed synthesis of dibenzo[18]crown-6 in 48 % yield. This breaking news, coming out of the Dupont Laboratories in Delaware, flew in the face of all the teaching I had experienced as an undergraduate student at Edinburgh, where I had been led to believe that, while making five-, six-, and seven-membered rings was commonplace, large-sized rings were a totally different kettle of fish. I also realized that these macrocyclic polyethers—or crown ethers as Pedersen had called them—shared some of the constitutional features (OCCO repeating units) with the sugars, and so I set off on a mission to pursue what I referred to as ‘lock-and-key chemistry’ by simply marrying conceptually Pedersen's crown ethers with Emil Fischer's carbohydrates. Of course, it was easier said than done, for I was only one pair of hands with many other things on my mind. One of them was to return to Edinburgh in the Fall of 1968 to say goodbye to the farm—for my parents had decided that after my leaving for Canada it was simply too much for them to handle on their own—and the other was to get married in Glasgow in the presence of close family members to Norma on 8th October 1968. We returned to Canada the next day via Montreal, my newly wed wife occupying her time during the flight by completing mountains of immigration paper work. At the airport we were greeted by a custom's officer who took one look at us, summed up the situation, crumpled the papers into a ball, threw them into a waste-paper bin (trash can) with the words “we grow trees in Canada and far too many of them get turned into paper” and waved us through to begin our married life in a foreign country with a welcome we were never to forget. Perish the thought that such a welcome would occur at an international border in today's world.
Our remaining 15 months at Queen's were blissful ones. We lived at 432 Alfred Street after I had negotiated to rent the house from the owners, Thelma and Dave Buchan, who had more or less become my Canadian ‘aunt and uncle’ during my first 18 months as a boarder in their home. Norma had completed research for her PhD degree, like myself in just over two years, but not without a never-to-be-forgotten incident following the decision that I would type the manuscript on my portable Olivetti typewriter. It was approaching midnight and I was typing the last few pages of her thesis. Norma decided I needed a cup of coffee and duly set the cup down on the table next me. The next time I triggered the carriage return it hit the cup fair and square on its side and propelled most of the contents right over the stack of 150 typed pages. Norma retired to a corner of the room sobbing her heart out. After a kiss and a cuddle I sent her off to bed and then stayed up all night, retyping much of the thesis by the following morning. When disaster strikes, it is best to waste no time in putting the experience to rest.
During my stay at Queen's I found it easy to interact with the faculty. Saul Wolfe, in particular, took me under his wing and transmitted to me the importance of being on top of the current literature. He brought to my attention the teachings of Kurt Mislow at Princeton on the importance of applying molecular symmetry to stereochemistry. Mislow had just introduced the concept of topism for analyzing the topic relationships between atoms and ligands in molecules. Amongst other attributes, it rendered the interpretation of NMR spectra a much easier task and helped to save me the embarrassment of coming to a wrong conclusion more than once. I had the opportunity to travel down to Princeton with Saul to meet this sage of stereochemistry. There were other opportunities to listen to lectures by the intellectual leaders of their time in organic chemistry, among them the famous Harvard professor, and synthetic chemist par excellence, R. B. Woodward, whom I recall holding an audience in the palm of his hand in Ottawa for more than three hours. Then, the Queen's chemistry department invited Saul Winstein from the University of California at Los Angeles (UCLA) to give the MacCrae lectures in the Spring of 1969. Winstein was considered by many to be the intellectual leader in physical organic chemistry at that time and would almost certainly have been the recipient of a Nobel Prize in Chemistry, had he not died very suddenly of a heart attack, at age 57, in November of that same year. What I recall most vividly about the MacCrae lectures was the manner in which Winstein launched into a 20-minute diatribe against H. C. Brown, reflecting the bitter controversy that raged between them for years over classical (HCB) versus non-classical (SW) carbocations. I could not have known in 1969 that almost 30 years later I would be making my way to UCLA to become the second holder of the Winstein Chair, following Donald Cram who shared the 1987 Nobel Prize in Chemistry with Charles Pedersen and Jean-Marie Lehn from the University of Strasbourg.
Saul Wolfe was a pupil of the highly influential and renowned carbohydrate chemist, Ray Lemieux, for whom I had acquired an enormous respect after hearing him give a series of remarkable named lectures (Purves, if I recall correctly) at McGill University, which ultimately led me to write a monograph on the “Stereochemistry of Carbohydrates”. I set out on this mission with the support of Ken Jones, who had returned from Brazil, without realizing the responsibility one assumes when writing a book! My attendance at a symposium hosted by the US Army Laboratories at Natick led to my meeting Ernest Eliel, the author of “The Stereochemistry of Carbon Compounds,” a classic published by McGraw–Hill in 1965. It had been my bible from my Edinburgh days and so I decided that I would approach Dr Eliel at the end of his inspirational talk and ask him if he would be kind enough to look over and comment on my manuscript. I sent him the manuscript and within a very short space of time it came back plastered in red ink. This experience taught me that having my manuscripts scrutinized by experts wherever possible would save me no end of embarrassment in the fullness of time. On this occasion, no doubt, Ernest saved my bacon: he and his wife Eva were to become close friends of myself and Norma for the rest of their lives.
Sheffield in the Seventies
As the 1960s came to a close, Norma convinced me that it was time to return to Old Blighty where we would give some thought to raising a family. Sometime in the summer of 1969 Ken Jones came back from a conference in the Caribbean with the news that David Ollis from Sheffield had given a lecture (with demonstrations) on the conformational behavior of a 12-membered ring compound known as tri-o-thymotide, or TOT for short, that had captured everyone's imagination. I decided to apply for an ICI Fellowship to go to Sheffield but initially failed to make the cut. Three months later I heard the good news that I had, after all, landed this prestigious fellowship, as one of the successful candidates had decided not to accept the offer. We decided it would be practical to ship our goods and chattels across The Pond and enjoy an ocean liner experience onboard the West German flagship Bremen during the week before Christmas. Five days after leaving New York we arrived in Southampton to be greeted by thick fog, which made the drive north to Edinburgh, stopping off in Sheffield on the way, all the more challenging.
There were several reasons for going to Sheffield. One was to attend the Annual Sheffield Stereochemistry Meeting, where I had the opportunity to hear Jean-Marie Lehn speak for the first time. It was such a pleasure to listen to this young French chemist with a research agenda in the making that was destined to chart new territory for the subject beyond the molecule or, as Lehn named it subsequently, supramolecular chemistry. Another reason for being in Sheffield was to introduce myself to David Ollis. When the subject of my start date came up he insisted I should be present in the department for the 1st of January 1970. This edict infuriated Norma, and I was not best pleased either, given the fact that we were heading to Scotland where New Year's Day is a national holiday. The crossing of swords with Ollis would go on for the best part of two decades.
On my return to the chemistry department on 1st January it became apparent that I was not going to be allowed the independence to carry out the kind of research that was the fellowship's official remit. In addition, when Ollis learnt that I would be spending some of my time writing the final chapter of the book, he immediately expressed his displeasure, stating quite emphatically that “people at my stage should not be writing books.” Norma, who was illustrating the manuscript with India ink and stencils, convinced me to ignore his decree and the monograph was published in 1971 by Wiley. If the welcome to Sheffield was muted from on high, Norma and I were made to feel very welcome by the postgraduate community, particularly by David (Dave) Brickwood (whom I was delegated to supervise), Stephen (Steve) Potter and Richard (Dick) Taylor. Little do they really know how much they helped us through those difficult times.
I was working in my laboratory (E19) on Good Friday in 1970 when Ollis walked in to tell me that at a meeting of the Organic Staff the day before, it had been decided that I should be offered a Lectureship in Chemistry—a position that had unexpectedly fallen vacant with the resignation of the youngest member of the staff—from 1st October. I was, of course, happy to have some long-term job security, although it in no way earned me my independence. It was 1973 before Andrew Coxon became my first independently supervised postgraduate student. For my first lecturing assignment I was handed a poisoned chalice in the shape of teaching the first-year medical students (all 180 of them) organic chemistry, in the knowledge that the course would soon be discontinued. The refrain from the students was very much along the lines of “Why are we having to take this course when it's about to be withdrawn?” It was a tall order to hold their attention in lectures and laboratory classes, but I did my very best to engender their enthusiasm by introducing all sorts of innovations into my teaching. Nonetheless, when brought before a group of medical staff in the presence of their dean, I was informed by him that “we might as well be teaching our students biblical studies.” It was a crushing put-down but I reasoned that I should not have been the person from the chemistry department finding himself in this particular lion's den!
Despite the fact that my progress in research was being forestalled at every turn by the antics of the professors in the department, Andrew Coxon, and later Dale Laidler, made some notable advances in their research with carbohydrate precursors to crown ethers, to the extent that when I was invited to speak at international conferences and symposia I had some interesting results to talk about under the banner of ‘lock-and-key chemistry’. A major turning point in my fortunes came in 1976, when I was invited to give no less than 17 lectures and seminars, nine in the UK, including Oxford, Imperial College London, Edinburgh and Glasgow, four in the US, including Columbia, Princeton and Dupont, and four in Canada, including McGill and Queen's. I was also fortunate in being invited to give a talk at the Centennial American Chemical Society Meeting in New York in early April. This invitation afforded me the opportunity to listen to Donald Cram speak and to meet with him one-on-one—along with his shopping bag full of CPK space-filling models—for the first time. Once again I found myself in the company of an eminent American chemist, who not only enthused about his own research, but also about mine, an experience for me that was uplifting beyond my wildest dreams. Don was also the RSC Centenary Lecturer in May 1976. He insisted that I would be one of the supporting speakers in Manchester and, two days later, in London, at University College. When the powers that be at the RSC questioned my double act, Don swept aside their protestations with the comment that “apart from Fraser and myself, those in the audiences in the two places will be different” and, of course, no one could argue with him, for he was right! David Ollis was livid but Don was drawing considerable satisfaction from the situation because he knew how I was being treated on home turf. Don also presented his Centenary Lecture in Sheffield and went out of his way to say he had come because of my presence in the department. He went on to lavish praise on my research group, leaving Ollis red with rage!
During these meetings, Don encouraged me to apply to the Science Research Council (SRC) for a Senior Research Fellowship to spend the first three months of 1978 on sabbatical leave at UCLA. This short stay in the UCLA Department of Chemistry and Biochemistry was a real breath of fresh air and served to increase my yearning to move to the US one day in the future. Interest in hiring me had been mooted in a number of different US universities, but then something else happened in the UK that I could live with very comfortably, and that left Norma happy that our two girls, who had arrived on the scene in 1973 (Fiona) and 1976 (Alison), could continue their education in the UK. That development involved the SRC, who were ready, willing and able to support my secondment to the ICI Corporate Laboratory in Runcorn, under the auspices of a brand new Cooperative Research Scheme for three years, from 1978 to 1981. It also received the backing of a number of ICI's senior management, including Tom McKillop and Bernard Langley. I was over the moon. I was free at last to carry out my own research in a highly supportive and amazingly well-equipped environment, staffed with research scientists who were second to none. We sold our home on Derriman Avenue in Sheffield and moved across the Pennines to a brand new house in Curzon Park in Chester, with a six-month layover in a small rented property in Little Sutton, on the Wirral. The next three years were amongst the happiest that we spent as a family in England.
Runcorn
I joined Warren Hewertson's Catalysis Group at ICI's Corporate Laboratory and supervised a couple of postgraduate students in Runcorn, plus half a dozen who remained in Sheffield, where I spent minimally one day a week. The Corporate Laboratory, situated on The Heath at Runcorn, was probably the closest one could get to a Bell Laboratories experience in the UK. It was in this setting that I quickly struck up a highly productive collaboration with a brilliant young chemist, Howard Colquhoun, who had only recently joined the laboratory. Following some discussions about what different kinds of complexes could be formed with crown ethers, we came to the conclusion that, as far as we knew, transition metal ammines had not been put to the test. We were fortunate insofar as there was a treasure trove of these ammines down in the basement of the laboratory that had been prepared by Joseph Chatt when he was an employee of ICI during the 1950s. I could not believe our luck. Before long we had lots of crystals of adducts of transition metal ammines with crown ethers, whose solid-state superstructures were solved at the drop of a hat by David Williams, X-ray crystallographer extraordinaire, down in London at Imperial College.
Amidst all these many superstructures, one caught our attention. It was the 1:1 adduct in which dibenzo[30]crown-10 (DB30C10) wraps itself round a dicationic platinum complex, carrying a 2,2′-bipyridyl ligand in addition to a couple of cis-diammine ligands, in such a manner that the ammine ligands form hydrogen bonds with the polyether loops of the crown ether, while the two π-electron rich catechol units sandwich the π-electron deficient bipyridyl ligand in a stacking manner. The structural similarities between this bipyridyl ligand and the bipyridinium herbicide Diquat (DQT) was pointed out to us by former ICI research scientist Eric Goodings. Sure enough, when the transition metal complex was replaced by DQT we obtained deep orange crystals of a 1:1 complex with DB30C10, as revealed yet again by its solid-state superstructure. Both the adduct and the complex, when associated with soft counterions, are reasonably stable in acetonitrile solution, as indicated by the presence of diagnostic charge-transfer bands that render the solutions light yellow and bright orange, respectively.
We had injected new life into Alfred Werner's concept of second-sphere coordination in the process of establishing donor–acceptor interactions as a force to be reckoned with in molecular recognition processes. They would ultimately serve as the sources of templation in the making of molecules with mechanical bonds. Although we had still to address the need to form complexes between crown ethers and Paraquat (PQT)—the other component of the wipe-out weed-killer that ICI marketed worldwide for many years—we had given the search for the ‘big problem’ an enormous fillip from an unlikely starting point. If I had not spent those years at ICI's Corporate Laboratory, my role in the development of mechanically interlocked molecules, that has led to designing and synthesizing molecular machines, would either not have happened or would have taken a very different course. All of what I was subsequently to achieve in research can be traced back to these three years.
I left Runcorn in the late summer of 1981 with a heavy heart, but there was no option. My three-year secondment was coming to a close and, more disturbingly, the writing was on the wall for the Corporate Laboratory. Norma and the girls had come to enjoy life in Chester and it was going to be challenging for all of us to return to Sheffield. Once again, the transfer was staged by my acquiring a small semi-detached home in Bradway, from which we were able to purchase the ideal family home in the shape of an Edwardian house on Dore Road.
Sheffield in the Eighties
My situation at Sheffield had been strengthened by my industrial experience and I was promoted to a Readership in Chemistry in 1982. Although many of the same issues still existed in the chemistry department at Sheffield, I was much more able to handle the slings and arrows of outrageous fortune. With growing confidence, I became quite vocal at the national level about the weaknesses, as I saw them, in the British academic system. My pronouncements and my writings—often to the British national newspapers—did not win me many friends, but at the same time they served to define where I stood on a wide range of issues. Eventually those in influential positions started to notice and take note.

With Norma outside our third Sheffield home in Bradway c. 1982. Inset: After graduating from Edinburgh in 1980 with a DSc degree.
At this time I struck up another important relationship that not only turned out to be of immense value in the promotion of my research as it developed during the 1980s, but also helped me launch some university-wide initiatives, such as the Sheffield Industrial Forum in 1986. That relationship was with Roger Allum, the Press Officer for the University. He was extremely supportive and would always seek to make our research intelligible to the wider public. If I ever felt a little depressed from working in a department that was brim full of politics, I could take a walk up to the Edgar Allen Building and have a reassuring chat with Roger. He always had time for me, no matter how busy he was tending to other University business. I would leave his office with my spirits lifted and ready to take on the world.
As I moved from one university to another, the importance of maintaining good and close relationships with the talented individuals in media relations remained with me. Martin Hicks at Birmingham continued in the footsteps of Roger and once I reached the University of California, Los Angeles (UCLA), I was to learn a lot from Stuart Wolpert on how to handle live and recorded interviews for radio and television. At Northwestern University (NU) I have been blessed many times over to have Megan Fellman working closely with myself and members of my research group in getting story after story out into the public domain. More recently, I have discovered a soulmate in Stephanie Russell, Editor of the Northwestern magazine, who has gone to considerable lengths, and well beyond the call of duty, in presenting me and my research to the alumni and friends of NU, following my award of the Nobel Prize in Chemistry.
On the scientific front, after some wasted effort and unproductive years, we were able to demonstrate quite simply that a constitutional isomer of DB30C10, namely bis-para-phenylene[34]crown-10 (BPP34C10), forms a strong 1:1 complex with PQT. The fact that the solid-state superstructure of this complex was ‘rotaxane-like’ in its appearance led me to suggest that it be called a [2]pseudorotaxane, a name which eventually transmogrified into meaning a template that could subsequently be converted into a catenane as well as a rotaxane. We had established that we could thread a π-acceptor through a ring containing two laterally disposed π-donor units. Our next challenge was to reverse this recognition motif by making a cyclophane in the form of cyclobis(paraquat-p-phenylene) and containing a couple of parallely disposed bipyridinium units held rigidly apart at a plane-to-plane separation of approximately 7 Å by two para-xylylene units, through which π-donors of many different persuasions could thread. In the first instance, Mark Reddington was able to prepare this cyclophane starting from 4,4′-bipyridine and xylylene dibromide in a 12 % yield.
During our efforts to publish the synthesis and full characterization of this cyclophane in Angewandte Chemie, I received a curt letter from Siegfried Hünig at the University of Würzburg, explaining that one of his students had synthesized a whole range of very similar cyclophanes and studied their ability to complex aromatic hydrocarbons, a piece of information that was available, but overlooked by me, in Dissertation Abstracts. I wrote back to Professor Hünig, who had clearly been one of the reviewers of our communications, and suggested that he write up a communication on his work while we delayed the publication of our communications so that all three could appear in the journal in a row. Thereafter, Siegfried and I became close friends, to the extent that he and his wife invited Norma and myself to Würzburg to help celebrate his 80th birthday in 2001. The publication of our two communications coincided with the beginnings of my use of color—red for π-donors and blue for π-acceptors—so that the cyclophane soon became known in the literature as the ‘little blue box’ and was to gain considerable notoriety as a promiscuous host for a wide range of π-donors, including benzidene and tetrathiafulvalene. Subsequently, employing both templates and catalysts—and some other tricks—we have been able to prepare the little blue box in all but quantitative yield.
The stage was now set to carry out the template-directed synthesis of the first donor–acceptor [2]catenane in a remarkable 70 % yield, by very simply employing the ingredients used in the preparation of the little blue box in acetonitrile at room temperature in the presence of three molar equivalent of BPP34C10. This experiment, which was carried out by Cristina Vicent and Neil Spencer, was one of the most memorable as we all gathered to watch the reaction mixture turn orange and crystals start growing on the side of the reaction flask within 10 minutes. I realized there and then that we were sitting at the entrance of a gold mine as we prepared the manuscript for publication in Angewandte Chemie in October of 1989. While the manuscript was out for review, I received a phone call from Jean-Pierre Sauvage in Strasbourg saying how impressed he was by the contents and offering me his congratulations. He was obviously one of the reviewers.
The 1980s represented a sea change for my group, as I began to realize that our level of research performance could be raised out of all recognition by welcoming postgraduate students and postdoctoral fellows from overseas. The arrival of Franz Kohnke from the University of Messina, not to mention the short visit of Cristina Vicent from Madrid, had a profound effect on the group culture as we became increasingly international in our composition. The cultural change also encouraged home-grown PhD students to raise their sights. Following graduation with their PhD degrees, David Leigh went to Ottawa in search of postdoctoral experience with David Bundle, while John Mathias, equipped with a postdoctoral fellowship, was invited by George Whitesides to go to Harvard.
Pier Lucio Anelli, who came to Sheffield as a postdoctoral researcher from the University of Milan, was another of a growing number of makers and shakers. Employing a pre-prepared dumbbell-shaped molecule as a template, he synthesized by templation a degenerate [2]rotaxane with two π-donating, hydroquinone-based, recognition sites for encirclement by one little blue box, which could be shown by dynamic NMR spectroscopy to be darting back and forth between the recognition sites at around 2000 times per second. I called it a molecular shuttle and concluded in a 1991 JACS communication that it was “the prototype for the construction of more intricate molecular assemblies where the components will be designed to record, store, transfer and transmit information in a highly controllable manner following their spontaneous self-assembly at the supramolecular level.” The development of this next step in the research program had to wait until a move to the University of Birmingham had been planned and executed.
Birmingham
I had been approached in 1991 by the then Vice-Chancellor of the University of Birmingham, Sir Michael Thompson, to consider moving to Birmingham as the Professor of Organic Chemistry. He had been attracted by my refusal to join the large group of whingers in British academia at that time. The Department of Chemistry was in a badly run down state and morale was low to say the least. After much discussion and an undertaking by the Vice-Chancellor to implement a staged refurbishment of the Haworth Building and invest in some key state-of-the-art equipment, including NMR and mass spectrometers, I accepted the chair and started a phased move of my research group, now growing in size, from Sheffield to Birmingham. Norma remained in Sheffield to look after the everyday needs of the group members there, while I oversaw the revamping of the top (seventh) floor of the building and prepared for the new spectrometers to arrive on the scene. While Neil Spencer accepted the challenge of establishing the new NMR facility, I managed to persuade the highly gifted senior technician, Peter Ashton, to also make the move from Sheffield to Birmingham and establish a mass spectrometry facility that was second to none in the country. I commuted between Birmingham and Sheffield for more than a year, given the added responsibility of being one of the organizers, along with Norma and David Fenton, of the 1991 International Symposium on Macrocyclic Chemistry, at which both Donald Cram and Jean-Marie Lehn received Honorary Degrees in Science from Sheffield University. It was also the occasion when the first International Izatt–Christensen Award was presented to Jean-Pierre Sauvage.

With Don and Jane Cram at the 16th International Symposium on Macrocyclic Chemistry held at Sheffield University in September 1991.
A life-changing event was to occur in February 1992 when I took an early morning phone call in my Birmingham office from Alison, her first words being, “Something terrible has happened, Daddy.” She went on to explain that her mother was in hospital, having suffered a brain hemorrhage overnight. I wasted no time in jumping into my car and driving up to Sheffield, only to be told by the surgeon in charge of her case that he was going to have to operate and that there was no better than a 50 % chance that Norma would survive the surgery. It was a long day that was to take a turn for the better when the surgeon informed me in the early evening that the artery in Norma's brain had self-healed and he would not need to operate. Relief all round! We moved from our Edwardian home in Sheffield to a 1930s home in Edgbaston, close to the campus of the University of Birmingham, on 1st April. With Norma still very much in a convalescent state, I was approached by Ken Houk at UCLA, who asked me if I would consider moving to UCLA to assume occupancy of the Winstein Chair on the impending retirement of Don Cram. My reply—with mixed emotions—was an easy one. Norma was too ill for me even to share this news with her and I was in the throes of a complicated relocation. I assumed that my message to Ken declining his offer would be the last I would hear of the Winstein Chair at UCLA and that this tantalizing prospect had slipped out of my grasp. This assumption proved to be incorrect.
As far as research was concerned, my seven years at Birmingham were to exceed my wildest dreams. Our first bistable [2]rotaxane, that could be switched both chemically and electrochemically, reached the literature in 1994, following a sojourn by Richard Bissell at the University of Miami with Angel Kaifer. Olympiadane was self-assembed by David Amabilino, while Gunter Mattersteig synthesized the first bistable [2]catenane, in which the two π-donating hydroquinone recognition sites in the degenerate [2]catenane were replaced with tetrathiafulvalene and dioxynaphthalene recognition sites. A highly fruitful collaboration, in which this catenane and many other bistable MIMs were switched chemically, electrochemically and photochemically, was struck with Vincenzo Balzani and Alberto Credi at the University of Bologna. Jon Preece spent time in the laboratory of Helmut Ringsdorf at the University of Mainz learning how to produce Langmuir monolayers and films of both degenerate and bistable [2]catenanes and preparing the way for device fabrication when we reached UCLA in 40.

The research group at Birmingham c. 1995.
Douglas Philp was a major intellectual driving force in the group during its early days in Birmingham. Aside from his high level of productivity that matched his creativity every inch of the way, he left a considerable legacy by writing a much-cited review on “Self-Assembly in Natural and Unnatural Systems” that was published in Angewandte Chemie in 1996. While Peter Glink established hydrogen bond templation (known within the group as ‘ammonium binding’) as a means of templating the synthesis of MIMs, Jayaraman and Sergey Nepogodiev launched ambitious programs of research into glycodendrimers and the synthesis of cyclic oligosaccharides related to the cyclodextrins. Steven Langford and Matthew Fyfe took over where Douglas Philp left off by bringing their keen intellects and dedicated commitment to the development of MIMs to a highly sophisticated level in relation to their physical organic chemistry.
Unwelcome news kept breaking in 1992. In August, Norma was diagnosed with breast cancer and underwent surgery in the form of a lumpectomy, followed by radiation and chemotherapy. The cancer recurred two years later in 1994, resulting in a mastectomy and yet more of the inevitable back-up treatment. This did not halt the progress of the disease, which was diagnosed as having become metastatic in 1996. During a visit to UCLA in 1994 to participate in a symposium to mark Don Cram's 75th birthday, Ken Houk raised once again the availability of the Winstein Chair, reiterating the interest of the Department of Chemistry and Biochemistry in my coming to occupy it at UCLA. My feeling that Norma was not receiving the best of medical care in Birmingham was accepted by her in early 1997 and so we decided to go on a trip to the US, visiting the M. D. Anderson Clinic in Houston and the Jonsson Comprehensive Cancer Center at UCLA, where Norma was told by the oncologists we met that, while she had a chronic disease, they had 50 different ways of treating it. At this point it was decided that I would step down from being the Head of the School of Chemistry at the end of June and formally move to Los Angeles to take up the Winstein Chair on 1st July 1997. Some 15 members—including first-year graduate students Stuart Cantrill, David Fulton, Sarah Hickingbottom, James Lowe and Anthony (Ant) Pease—of my research group made the transition from the middle of England to the West Coast of America, with postdoctoral fellow Françisco Raymo acting out the role of the scout. Coming to grips with the very different way American academia operates compared with that in the UK, together with getting my mind round the funding system from the federal agencies and beyond, constituted a baptism of fire for a 55-year-old. We simply rolled up our sleeves and got on with it. Norma, for the first time gainfully employed as a research assistant to my group by UCLA, helped in all this.
University of California Los Angeles (UCLA)
In just over a decade at UCLA, from 1997 to 2008, we broadened the scope of our template-directed approaches to mechanically interlocked molecules (MIMs) by appealing to both hydrogen-bond and metal templation, as well as developing donor–acceptor templation to cover the production of a wide range of molecular switches. Stuart Rowan joined my research group in 1998, with the intention of establishing his own independent academic career in the United States, after having played a major role in the furtherance of dynamic covalent chemistry (DCC) at the University of Cambridge with Jeremy Sanders. This thermodynamically controlled approach to the template-directed synthesis of MIMs can be extraordinarily powerful. It eventually led to high-yielding syntheses of molecular Borromean rings and Solomon knots. Template-directed approaches under kinetic control to MIMs began to rely more and more on the use of ‘click chemistry’, as popularized by Barry Sharpless. Amongst the ring leaders during this period—in addition to Stuart Rowan (University of Chicago)—were Ivan Aprahamian (Dartmouth College), Adam Braunschweig (Hunter College), Sheng-Hsien Chin (National Taiwan University), William Dichtel (Northwestern University), Amar Flood (Indiana University), David Fulton (University of Newcastle), Jan Jeppesen (University of Southern Denmark), Steve Joiner (Moorpark College), Ken Leung (Hong Kong Baptist University), Cari Meyer (Pierce College), Ognjen Miljanić (University of Houston), Al Nelson (University of Washington), Brian Northrop (Wesleyan University), Hsian-Rong Tseng (University of California, Los Angeles), Bruce Turnbull (University of Leeds), Sebastian Vidal (University of Lyon), Scott Vignon (Washington DC) and Jishan Wu (National University of Singapore).
The UCLA era was characterized by numerous efforts to uncover applications for molecular switches, both the non-degenerate catenated and rotaxanated varieties. One of the most rewarding and fulfilling collaborations was with Jim Heath in the field of molecular electronics. The marriage between molecular switches and electrodes is far from being an easy one, and I have to say that Jim picked his way through what turned out to be a bit of a minefield with the greatest of ease. By employing crossbar devices, he and his highly skilled team of graduate students and postdoctoral fellows were able, using the LB technique established during the Birmingham days, to lay down monolayers of switchable catenanes and rotaxanes between parallel wires of polysilicon (bottom electrodes) and orthogonally disposed parallel wires of titanium capped with aluminum. By 2007, using an amphiphilic bistable [2]rotaxane, a 160,000-bit molecular electronic memory circuit had been fabricated at a density of 100,000,000,000 bits per square centimeter. The entire 160-kbit crossbar device was smaller than the cross-section of a white blood cell. It transpired that there is one fatal weakness with the crossbar devices, and that is their lack of robustness. When Omar Yaghi arrived at UCLA in 2006 we started a joint program of research, whereby bistable MIMs are being incorporated inside metal–organic frameworks—and it continues today at Northwestern University (NU) in collaboration with Joe Hupp and Omar Farha.
For a time Norma's oncologists kept her cancer at bay, chiefly by moving in the face of resistance to treatment from one anticancer drug to another, and subsequently to a cocktail of two or three or more of them. She and I were able to travel the world together for a while, visiting many cities, including Paris, Stockholm and Vienna in Europe and Kyoto and Nara in Japan. Slowly and perceptibly, Norma's state of health started to wane as the side effects of the drugs began to sap her energy, causing her to seek refuge in our small Santa Monica townhouse, assisted by a kind and marvelous caregiver, Sylvia Mena, and no end of material and psychological support from Alice Jung, wife of my colleague Mike Jung, who was a dab hand at making Norma laugh and in so doing lifting my spirits. She referred to Mike's other half as ‘Alice the Angel’. By late November 2003, the 25th to be precise, Norma's head oncologist, John Glaspy, told me what I had already guessed: it was that the disease had reached her brain and that it was only a matter of time, a few weeks at most, before a battle, that had occupied a fifth of her life and demanded our attention for a third of our married lives, was about to end. Norma always insisted that her brain was her last refuge: if and when it was invaded by the ‘little buggers’, she would throw in the towel. Her final foray into the outside world was a sight to behold. It was an excursion to Gap in Santa Monica to purchase a large selection of garments for her grandson, only a few weeks away from being born to Fiona and Quentin McCubbin, yet she was not going to set eyes upon James Fraser (the Second!). Norma's shopping sprees were legendary, but this one stole the show. For the first time since 1966, she was oblivious to the spirit and trappings of Christmas as she prepared to make a dignified exit, simply commenting that she had drawn the short straw. During the final days of her life she communicated with me using a pencil and writing pad, being too weak to speak. Her last comment, written the night before she passed away on 12th January 2004, was, “Am I dead yet?” She sank into oblivion as I was struggling to decipher her question and so I was not able to provide her with an answer. In the last few weeks of her life she was insistent that her main legacy were ‘her girls’ and there is no arguing with that statement to this day. She had every right to feel proud of Fiona and Alison.
The departure in 2003 of Jim Heath to the California Institute of Technology (CALTECH) signaled two changes in my professional life. One was the taking over of the Directorship of the California NanoSystems Institute (CNSI) from Jim, the founding director, first of all in an acting capacity and then subsequently for real. Despite these developments, Jim and I maintained our collaboration in the realm of molecular electronics, aided and abetted by Bill Goddard's entry into the program. Through his impressive computational investigations, he did much to vindicate our proposed switching mechanism exhibited by monolayers of bistable rotaxanes in crossbar devices. I became a great admirer of Bill's command of his science and the fearless manner in which he tackles large and complicated problems, a trait that continues to this day. No one knows and understands our donor–acceptor catenanes and rotaxanes all the way from bistable molecules through to devices better than Bill: this belief is supported by arguments presented in more than 30 joint publications. Another positive change that occurred around 2003 was the forging of a close and equally fruitful collaboration at UCLA with Jeff Zink, whose knowledge and practical expertise in relation to the preparation of mesoporous silica nanoparticles led to the covering of the surfaces of these 100–200 nanometer diameter particles with both bistable/switchable rotaxanes (nanovalves) and their supramolecular counterparts, which we called snap-tops, as a sophisticated means of controlling the release of small molecules, such as anticancer drugs. My foray into drug delivery systems was undoubtedly influenced by my day-to-day experiences of living for 12 years with a cancer patient. I have to admit, however, that I am coming to the opinion, after having co-authored more than 30 articles with Jeff, that, while more and more sophisticated ways of delivering drugs to patients suffering from degenerative diseases can prolong their lives, they are probably never going to provide the cures that many people would like to think are just around the corner.
After graduating with his PhD from UCLA in 2001, Stuart Cantrill spent a couple of years at CALTECH as a postdoctoral scholar with Bob Grubbs, one of the 2005 Nobel Laureates in Chemistry. The outcome of this association was yet another collaboration, in which Grubbs-catalyzed olefin metathesis in many of its different manifestations was introduced into the thermodynamically controlled syntheses of MIMs using hydrogen bonding as the source of templation. Stuart returned to UCLA in 2003 to take on my undergraduate teaching responsibilities and to assist me in the running of my research group while I was CNSI Director. During what turned out to be a three-year sojourn, he also became the de facto Associate Editor of Organic Letters. It was during this time that he not only made sure that the research he had initiated in relation to the dynamic synthesis of the molecular Borromean rings reached the light of day in the literature, but he was also to discover that his own future lay in scientific publishing. On his return to the UK in 2005 he found employment with the Nature Publishing Group, first of all as an Associate/Senior Editor with Nature Nanotechnology, before being given the responsibility in 2008 to launch Nature Chemistry as its Founding Chief Editor. It is this kind of career progression by my students that I look back upon with immense pride.

Norma and I with David Leigh, Stuart Rowan and Stuart Cantrill after the International Symposium on Macrocyclic Chemistry held at St Andrews University in July 2000.
Two bolts appeared out of the blue in late 2006 and early 2007 that could be considered as serious game-changers. The first one arrived in the context of a phone call on 13th November 2006 from Bob Pierce, the British Consul General in Los Angeles. To my consternation, Bob asked me if I would be prepared to accept an appointment to Her Majesty the Queen as a Knight Bachelor. My acceptance, under a cloak of secrecy, became public knowledge in the 42 New Year Honours List. It led to my attending an investiture in June 2007, accompanied by David Leigh, a 1987 PhD graduate from my Sheffield days, along with Fiona and Alison. The second great surprise came in the form of a call to my cell phone from my assistant, Christina Oliver, while I was attending the Third Annual FENA Review Meeting at the LUXE Hotel in Los Angeles. On this occasion, the message, which encroached upon a conversation I was having with Youssry Botros (Intel), who was appointed as a consultant from industry to the Center for Functional Engineered Architectonics (FENA) directed by Kang Wang at UCLA, was from the King Faisal Foundation in Riyadh to say that I had been selected to receive the 2007 King Faisal International Prize (KFIP) in Science. Youssry, who was born and brought up in Egypt, was immediately raised to a highly excited state on learning the news from me. In the event, I invited Youssry, a fluent Arabic speaker, to accompany Alison, her then fiancé Mikey Ho, and myself on our first trip to Saudi Arabia to receive the Prize from the King in the middle of April. During a week-long visit, Youssry and I had our first meeting with Prince Turki Al-Saud who was then Vice-President of the King Abdulaziz City for Science and Technology (KACST), and he is now the President of KACST. Following this meeting, KACST has generously funded six projects at Northwestern University related to energy storage, energy harvesting, molecular electronics, porous materials, membrane technology and drug delivery, under the auspices of a Joint Center of Integrated Nanosystems (JCIN). Managing JCIN along with my highly supportive co-Director Majed Nassar, has been aided and abetted in a big way by Alyssa Avestro, Ashish Basuray, Tracy Chen and Mark Lipke.

Top: Three peas in a pod. With Alison and Fiona at Fiona's wedding in June 2000. Bottom: Outside Buckingham Palace with Alison and Fiona in June 2007 after being knighted by HM Queen Elizabeth.
It became clear towards the end of 2006 that the fortunes of the CNSI were set to suffer as a result of a change in the State of California Administration in Sacramento. Although buildings were nearing completion at both UCLA and the University of California, Santa Barbara (UCSB), it was apparent that there would be next to no funds made available from the State to equip the two buildings. I had little desire to find myself in charge of two white elephants.
I had tried to interest Chad Mirkin at Northwestern University in moving to UCLA to take over the Directorship of the CNSI from me. He was not interested but then turned the tables on me by inviting me to move to NU. Negotiations with the then President Henry Bienen at NU began in February 2007 and proceeded at such a pace that an announcement of my move could be made in August of that same year, allowing a few members of my group to start relocating a month later into newly refurbished laboratories in the Technological Institute. Although it was to take until August 2014 for a brand new building, sanctioned and supported on my advice by the President, to house some of the major items of departmental equipment (spectrometers and diffractometers) to materialize, it was well worth the wait. I moved up to Evanston on 1st January 2008 amidst a spate of gong-collecting. It seems that awards and prizes feed off one another to a considerable extent. In January 2010 my research group, under the guidance of Doug Friedman, moved with military-like precision from the Tech building into the newly opened Silverman Hall to occupy research space second to none in our previous 40-year history. It did not take long for it to be called the Research Palace—or RP for short.
Northwestern University
At Northwestern, a decade of broadly based activity in research relating to supramolecular chemistry, as well as mechanostereochemistry, has relied heavily on simply allowing a team of extremely creative graduate students and postdoctoral scholars free rein within the remit of the grants that support their research. This approach to invention and innovation in research has been highly successful, leading to a host of serendipitous events. One of these accidental discoveries, by Ron Smaldone—who was joined on its realization by Jeremiah Gassensmith and Ross Forgan—relates to the unexpected ability of γ-cyclodextrin to form highly porous extended structures with Group IA metal cations, particularly potassium, rubidium and cesium ions. This discovery in turn has led to the establishment of a start-up company, PanaceaNano, in 2010 with Youssry Botros as its Chairman and Chief Executive Officer (CEO). The company has developed several Organic Nano-Cube (ONC) based materials that are completely safe for use in many industries, such as cosmetics, home and personal care, health and medicine, chemical, environment, food and beverage, and agriculture. In less than one and a half years, the company has developed and shipped many prototypes for testing by collaborators and distributors in the cosmetics, fragrances and drugs areas. The other chance discovery, by Zhichang Liu, relates to a remarkable lock-and-key fit between α-cyclodextrin and potassium tetrabromoaurate in a 2:1 ratio within a linear and rigid supramolecular polymer, which aggregates in its thousands—like drinking straws in a box—to form needle-like crystals within minutes in aqueous solutions. A start-up company, Cycladex, was launched in 2014 with Roger Pettman, one of my early postgraduate students from my Sheffield days, as its CEO. The company is offering the opportunity to the gold-mining industry to abandon the use of cyanide and mercury in the isolation of gold from ore and to adopt a much less expensive environmentally friendly way of achieving a better outcome.
In the realm of supramolecular chemistry, the trio of Michal Juríček, Jonathan Barnes and Edward Dale devised much more user-friendly and efficient approaches to the synthesis of the little blue box, before going on to expand the dimensions of this tetracationic cyclophane to yield much larger receptors they called ExBox and ExCage, which turned out to be ideal for complexing polycyclic aromatic hydrocarbons. An intellectually satisfying piece of research carried out in collaboration with Jay Siegel at Tianjin University was the induced-fit catalysis of corannulene bowl-to-bowl inversion, which illustrates very nicely the principles of enzyme catalysis. In what is a simple textbook example, catalysis of the inversion process in corannulene, induced by its stereoelectronic binding inside ExBox, can be followed along a single ‘reaction’ coordinate, where the reactant and product are the same. A full paper published in the Journal of the American Chemical Society on ExCage—one of the shortest titles ever for an article on chemistry—amounts to a tour de force in contemporary physical organic chemistry enacted by the ExBox/ExCage trio.
The reason that the laissez-faire approach to supervising graduate students and postdoctoral scholars in the Northwestern chemistry department thrives so well is because it is an approach that is endorsed to the full by the vast majority of the faculty. The experimentally and computationally active members in different research groups interact with each other so well, more often than not from the bottom up, that it has led to the comment that we hunt in packs in the Department of Chemistry at Northwestern. Allowing these interactions to take their own course without meddling or interference is an extremely effective dynamic when it comes to the attainment of high-quality research. It is a dynamic that, is by and large, lost on university administrators and the regulatory authorities, who are much attracted and enamored by the concept of research being performed in silos. The fact that the laissez-faire approach is the dominant practice in the Northwestern Chemistry Department despite the presence of rules and regulations that would dictate otherwise, has rendered it possible for my research group personnel—Gokhan Barin, Florian Beuerle, Ali Coskun, Marco Frasconi, Sergio Grunder, Chenfeng Ke, Severin Schneebeli, Cory Valente and many others—to collaborate with their counterparts in the groups led by Mike Wasielewski, Joe Hupp, Omar Farha, Bartosz Grzybowski, Emily Weiss, Chad Mirkin, Mark Ratner, George Schatz and Lin Chen, while maintaining active collaborations with Bill Goddard's group at CALTECH and Omar Yaghi's group at UC Berkeley. Add to this list the name of my fellow Nobel Laureate Jean-Pierre Sauvage, who spent a couple of years (2010–2012) coming from Strasbourg to Evanston from time to time as a visiting professor, and you have yet another source of intellectual stimulation par excellence. His overarching presence encouraged us all to spend a considerable amount of time thinking deeply, practicing painstakingly and writing wisely about chemical topology, a subject area that will surely come into its own right in years to come.
Two additional developments at Northwestern deserve special recognition. One was the demonstration in 2010 by Ali Trabolsi, and followed through by Albert Fahrenbach, of the strong 1:1 complex formed between viologen radical cations and the bisradical dicationic cyclophane, obtained on reduction of the little blue box in the presence of methyl viologen or its derivatives. It was somewhat counterintuitive that three ‘free’ electrons would hold a complex together in the face of substantial Coulombic repulsion, but it is a fact! This discovery led to the template-directed synthesis of catenanes and rotaxanes. While Jonathan Barnes set about making the homo[2]catenane of the little blue box—an achievement which Diego Benítez described as being intellectually disruptive—Hao Li employed radical templation to make rotaxanes that have been introduced subsequently into the design and synthesis of a rapidly growing range of artificial molecular pumps by Chuyang Cheng, Paul McGonigal and Cristian Pezzato. This story is featured in my Nobel Lecture—produced, as in the case of hundreds of other presentations, with the help and expertise of graphic artist Alex Bosoy.
The other development worthy of special mention was the writing, along with Carson Bruns, of a major treatise on MIMs. In every respect—both words and pictures—the heavy lifting was done by Carson during a 30-month period that spilled over into some of his time as a Miller Research Fellow at Berkeley. It was quite fortuitous that Wiley ended up introducing the work to the world at large in the early part of November last year, halfway between the announcement of the 2016 Nobel Prize in Chemistry on 5th October and the prize-giving in Stockholm on 10th December. The manuscript was reviewed critically by many colleagues and the production of its six chapters, along with all the necessary components that go into the making of any book, were orchestrated by two people in particular. One was Xirui Gong, who read the proofs sentence-by-sentence, word-by-word, letter-by-letter, and number-by-number. The other was Margaret (Peggy) Schott, who assisted me in the demanding task of quality control as well as assuming responsibility for the production of the index in a highly efficient manner. Over the past 10 years at Northwestern University, Peggy has helped me, day-in and day-out, to guide a team of highly talented, yet often quite demanding, young researchers, from the day of their arrival to that of their departure and beyond into their own independent careers. These activities reflect only the tip of the iceberg when it comes to hailing the support Peggy—a PhD chemist and Northwestern alumna—provides to so many in the chemical community—locally, nationally and internationally.

Meeting President Obama in the Oval Office along with Fiona and Alison on 30 November 2016.

Signage. Top: The research group beside the Northwestern Arch in October 2016. Bottom: The front of the American Chemical Society Building in Washington, DC. In an e-mail received on 3 November 2016, Stu Borman of C&E News comments that “all I can see out my window now is ‘RAS’ and ‘DDA’”.

Top: Family gathered together at the Nordic Museum in Stockholm 2016. Bottom: In the midst of some young budding scientists at a party in the Grand Hotel in Stockholm 2016.
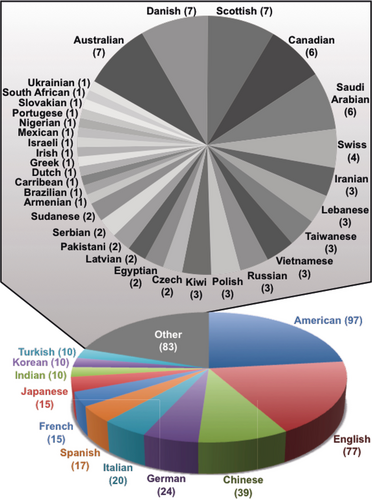
Breakdown of Stoddart group members during the past 45 years from 43 different countries. The data reflects the fact that, for those group members (a few) who have changed their nationality, they are counted twice, reflecting both their original and present nationalities, leading to a total number of entries of 417 on the pie charts. I thank Carson Bruns for producing this illustration at the drop of a hat!

Meeting Chinese Premier Li Keqiang in the Great Hall of the People on 20 January 2017.
Epilogue
In reflecting upon my peripatetic journey, which started with my valuable early experiences at the ‘University of Life’ on the farm, I can look back with feelings of pleasure interspersed with times of personal and professional hardships. There have been occasions marked by joy and others by sorrow. There have been periods that were characterized by success and others by failure, which I did my best to mitigate. Through all my life's experiences, the aim has always been the same: to emerge from life's roller coaster better informed and more knowledgeable about the ways of the world.
Putting all the ups and downs aside, I have been immensely privileged to be able to practice my hobby almost every day of my life in the presence of highly intelligent and outstandingly gifted young people, roughly aged between 18 and 32 drawn from nearly all quarters of the globe—and to do the things I love doing with them as a result of the generosity of those institutions and people, often without my being able to put a label or face to them, who have lent their support to my vision and mission from the Athens of the North (Edinburgh University) to the Windy City beside Lake Michigan (Northwestern University) with interludes on the edge of the Canadian Shield beside Lake Ontario (Queen's University), in the Socialist Republic of Yorkshire (University of Sheffield), on the Plains of Cheshire beside the Wirral (ICI Corporate Laboratory), in the Heartland of Albion (University of Birmingham and in the City of Angels alongside the Peaceful Sea (University of California, Los Angeles). My journey is far from over: it will continue as long as family and friends fail to raise a red card telling me that I have reached my sell-by date.
Science is global and there's no going back: scientists the world over live in a global village. There are no better words to catch this sentiment than those of the Scottish poet Robert Burns. In an epic poem, he emphasizes that “we're all the same under the skin.” It is a statement of egalitarian sentiments. The poem reads

Oh that people who exercise power and influence in the world over we ordinary mortals might be guided by these sentiments. What a wonderful world it would be for all humankind if there were no borders—and rejoice at the thought, no countries.