Synthesis of an Aluminum Hydroxide Octamer through a Simple Dissolution Method
Dr. Cory K. Perkins
Department of Chemistry, Oregon State University, 153 Gilbert Hall, Corvallis, OR, 97331-4003 USA
Search for more papers by this authorEric S. Eitrheim
Department of Chemistry, University of Iowa, Iowa City, IA, 52242 USA
Search for more papers by this authorBrantly L. Fulton
Department of Chemistry & Biochemistry, University of Oregon, Eugene, OR, 97403-1253 USA
Search for more papers by this authorLauren B. Fullmer
Department of Chemistry, Oregon State University, 153 Gilbert Hall, Corvallis, OR, 97331-4003 USA
Search for more papers by this authorChristopher A. Colla
Department of Chemistry, University of California, Davis, CA, 95616 USA
Search for more papers by this authorDeok-Hie Park
Department of Chemistry, Oregon State University, 153 Gilbert Hall, Corvallis, OR, 97331-4003 USA
Search for more papers by this authorProf. Anna F. Oliveri
Department of Chemistry, Southern Oregon University, Ashland, OR, 97520 USA
Search for more papers by this authorProf. James E. Hutchison
Department of Chemistry & Biochemistry, University of Oregon, Eugene, OR, 97403-1253 USA
Search for more papers by this authorProf. May Nyman
Department of Chemistry, Oregon State University, 153 Gilbert Hall, Corvallis, OR, 97331-4003 USA
Search for more papers by this authorProf. William H. Casey
Department of Chemistry, University of California, Davis, CA, 95616 USA
Search for more papers by this authorProf. Tori Z. Forbes
Department of Chemistry, University of Iowa, Iowa City, IA, 52242 USA
Search for more papers by this authorCorresponding Author
Prof. Darren W. Johnson
Department of Chemistry & Biochemistry, University of Oregon, Eugene, OR, 97403-1253 USA
Search for more papers by this authorCorresponding Author
Prof. Douglas A. Keszler
Department of Chemistry, Oregon State University, 153 Gilbert Hall, Corvallis, OR, 97331-4003 USA
Search for more papers by this authorDr. Cory K. Perkins
Department of Chemistry, Oregon State University, 153 Gilbert Hall, Corvallis, OR, 97331-4003 USA
Search for more papers by this authorEric S. Eitrheim
Department of Chemistry, University of Iowa, Iowa City, IA, 52242 USA
Search for more papers by this authorBrantly L. Fulton
Department of Chemistry & Biochemistry, University of Oregon, Eugene, OR, 97403-1253 USA
Search for more papers by this authorLauren B. Fullmer
Department of Chemistry, Oregon State University, 153 Gilbert Hall, Corvallis, OR, 97331-4003 USA
Search for more papers by this authorChristopher A. Colla
Department of Chemistry, University of California, Davis, CA, 95616 USA
Search for more papers by this authorDeok-Hie Park
Department of Chemistry, Oregon State University, 153 Gilbert Hall, Corvallis, OR, 97331-4003 USA
Search for more papers by this authorProf. Anna F. Oliveri
Department of Chemistry, Southern Oregon University, Ashland, OR, 97520 USA
Search for more papers by this authorProf. James E. Hutchison
Department of Chemistry & Biochemistry, University of Oregon, Eugene, OR, 97403-1253 USA
Search for more papers by this authorProf. May Nyman
Department of Chemistry, Oregon State University, 153 Gilbert Hall, Corvallis, OR, 97331-4003 USA
Search for more papers by this authorProf. William H. Casey
Department of Chemistry, University of California, Davis, CA, 95616 USA
Search for more papers by this authorProf. Tori Z. Forbes
Department of Chemistry, University of Iowa, Iowa City, IA, 52242 USA
Search for more papers by this authorCorresponding Author
Prof. Darren W. Johnson
Department of Chemistry & Biochemistry, University of Oregon, Eugene, OR, 97403-1253 USA
Search for more papers by this authorCorresponding Author
Prof. Douglas A. Keszler
Department of Chemistry, Oregon State University, 153 Gilbert Hall, Corvallis, OR, 97331-4003 USA
Search for more papers by this authorGraphical Abstract
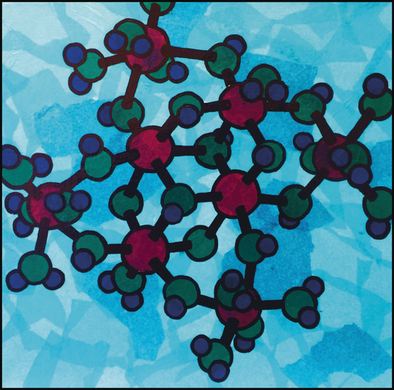
Super 8: The octameric aluminum hydroxide cluster [Al8(OH)14(H2O)18]10+ is readily synthesized and isolated through the dissolution of aluminum hydroxide in sulfuric acid and subsequent crystal growth. Structural studies reveal the existence of the cluster in solution. Al red, O green, H blue (Image by Dr. Anna Oliveri).
Abstract
Multimeric oxo-hydroxo Al clusters function as models for common mineral structures and reactions. Cluster research, however, is often slowed by a lack of methods to prepare clusters in pure form and in large amounts. Herein, we report a facile synthesis of the little known cluster Al8(OH)14(H2O)18(SO4)5 (Al8) through a simple dissolution method. We confirm its structure by single-crystal X-ray diffraction and show by 27Al NMR spectroscopy, electrospray-ionization mass spectrometry, and small- and wide-angle X-ray scattering that it also exists in solution. We speculate that Al8 may form in natural water systems through the dissolution of aluminum-containing minerals in acidic sulfate solutions, such as those that could result from acid rain or mine drainage. Additionally, the dissolution method produces a discrete Al cluster on a scale suitable for studies and applications in materials science.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201702318-sup-0001-misc_information.pdf711.3 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1W. Seichter, H.-J. Mögel, P. Brand, D. Salah, Eur. J. Inorg. Chem. 1998, 795–797.
10.1002/(SICI)1099-0682(199806)1998:6<795::AID-EJIC795>3.0.CO;2-A CAS Web of Science® Google Scholar
- 2W. Wang, K. M. Wentz, S. E. Hayes, D. W. Johnson, D. A. Keszler, Inorg. Chem. 2011, 50, 4683–4685.
- 3S. E. Smart, J. Vaughn, I. Pappas, L. Pan, Chem. Commun. 2013, 49, 11352–11354.
- 4G. Johansson, G. Lundgren, L. G. Sillén, R. Söderquist, Acta Chem. Scand. 1960, 14, 769–771.
- 5L. Allouche, C. Gérardin, T. Loiseau, G. Férey, F. Taulelle, Angew. Chem. Int. Ed. 2000, 39, 511–514;
10.1002/(SICI)1521-3773(20000204)39:3<511::AID-ANIE511>3.0.CO;2-N CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 521–524.
- 6S. Abeysinghe, D. K. Unruh, T. Z. Forbes, Cryst. Growth Des. 2012, 12, 2044–2051.
- 7W. H. Casey, M. M. Olmstead, B. L. Phillips, Inorg. Chem. 2005, 44, 4888–4890.
- 8K. S. Lokare, N. Frank, B. Braun-Cula, I. Goikoetxea, J. Sauer, C. Limberg, Angew. Chem. Int. Ed. 2016, 55, 12325–12329; Angew. Chem. 2016, 128, 12513–12517.
- 9G. Sposito, The Environmental Chemistry of Aluminum, CRC, Boca Raton, 1996.
- 10T. A. Stewart, D. E. Trudell, T. M. Alam, C. A. Ohlin, C. Lawler, W. H. Casey, S. Jett, M. Nyman, Environ. Sci. Technol. 2009, 43, 5416–5422.
- 11C. R. Armstrong, W. H. Casey, A. Navrotsky, Proc. Natl. Acad. Sci. USA 2011, 108, 14775–14779.
- 12D. A. Keszler, J. T. Anderson, S. T. Meyers in Solut. Process. Mater. (Ed.: ), John Wiley & Sons, Hoboken, 2009, pp. 109–127.
- 13R. P. Oleksak, R. E. Ruther, F. Luo, K. C. Fairley, S. R. Decker, W. F. Stickle, D. W. Johnson, E. L. Garfunkel, G. S. Herman, D. A. Keszler, ACS Appl. Mater. Interfaces 2014, 6, 2917–2921.
- 14J. W. Akitt, N. N. Greenwood, B. L. Khandelwal, J. Chem. Soc. Dalton Trans. 1972, 1226–1229.
- 15J. W. Akitt, J. M. Elders, J. Chem. Soc. Faraday Trans. 1985, 81, 1923–1930.
- 16J. W. Akitt, J. M. Elders, J. Chem. Soc. Dalton Trans. 1988, 1347–1355.
- 17M. N. Jackson, M. K. Kamunde-Devonish, B. A. Hammann, L. A. Wills, L. B. Fullmer, S. E. Hayes, P. H.-Y. Cheong, W. H. Casey, M. Nyman, D. W. Johnson, Dalton Trans. 2015, 44, 16982–17006.
- 18S. Goberna-Ferrón, D.-H. Park, J. M. Amador, D. A. Keszler, M. Nyman, Angew. Chem. Int. Ed. 2016, 55, 6221–6224; Angew. Chem. 2016, 128, 6329–6332.
- 19L. B. Fullmer, R. H. Mansergh, L. N. Zakharov, D. A. Keszler, M. Nyman, Cryst. Growth Des. 2015, 15, 3885–3892.
- 20A. F. Oliveri, E. W. Elliott, M. E. Carnes, J. E. Hutchison, D. W. Johnson, ChemPhysChem 2013, 14, 2655–2661.
- 21G. Johansson, Acta Chem. Scand. 1962, 16, 403–420.
- 22S. W. Smith, W. Wang, D. A. Keszler, J. F. Conley, J. Vac. Sci. Technol. A 2014, 32, 041501.
- 23C. K. Perkins, R. H. Mansergh, J. C. Ramos, C. E. Nanayakkara, D.-H. Park, S. Goberna-Ferrón, L. B. Fullmer, J. T. Arens, M. T. Gutierrez-Higgins, Y. R. Jones, J. I. Lopez, T. M. Rowe, D. M. Whitehurst, M. Nyman, Y. J. Chabal, D. A. Keszler, Opt. Mater. Express 2017, 7, 273–280.
- 24S. T. Meyers, J. T. Anderson, D. Hong, C. M. Hung, J. F. Wager, D. A. Keszler, Chem. Mater. 2007, 19, 4023–4029.
- 25K. Jiang, J. T. Anderson, K. Hoshino, D. Li, J. F. Wager, D. A. Keszler, Chem. Mater. 2011, 23, 945–952.
- 26J. T. Anderson, C. L. Munsee, C. M. Hung, T. M. Phung, G. S. Herman, D. C. Johnson, J. F. Wager, D. A. Keszler, Adv. Funct. Mater. 2007, 17, 2117–2124.