Synthesis of Long Oxahelicenes by Polycyclization in a Flow Reactor
Jindřich Nejedlý
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
These authors contributed equally.
Search for more papers by this authorDr. Michal Šámal
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
These authors contributed equally.
Search for more papers by this authorDr. Jiří Rybáček
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
These authors contributed equally.
Search for more papers by this authorMiroslava Tobrmanová
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorDr. Florence Szydlo
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorDr. Christophe Coudret
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorMaria Neumeier
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorCorresponding Author
Dr. Jaroslav Vacek
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorDr. Jana Vacek Chocholoušová
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorMiloš Buděšínský
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorDr. David Šaman
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorDr. Lucie Bednárová
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorDr. Ladislav Sieger
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Department of Physics, CTU in Prague, Faculty of Electrical Engineering, Technická 2, 16627 Prague 6, Czech Republic
Search for more papers by this authorCorresponding Author
Dr. Irena G. Stará
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorCorresponding Author
Dr. Ivo Starý
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorJindřich Nejedlý
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
These authors contributed equally.
Search for more papers by this authorDr. Michal Šámal
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
These authors contributed equally.
Search for more papers by this authorDr. Jiří Rybáček
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
These authors contributed equally.
Search for more papers by this authorMiroslava Tobrmanová
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorDr. Florence Szydlo
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorDr. Christophe Coudret
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorMaria Neumeier
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorCorresponding Author
Dr. Jaroslav Vacek
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorDr. Jana Vacek Chocholoušová
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorMiloš Buděšínský
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorDr. David Šaman
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorDr. Lucie Bednárová
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorDr. Ladislav Sieger
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Department of Physics, CTU in Prague, Faculty of Electrical Engineering, Technická 2, 16627 Prague 6, Czech Republic
Search for more papers by this authorCorresponding Author
Dr. Irena G. Stará
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorCorresponding Author
Dr. Ivo Starý
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6, Czech Republic
Search for more papers by this authorGraphical Abstract
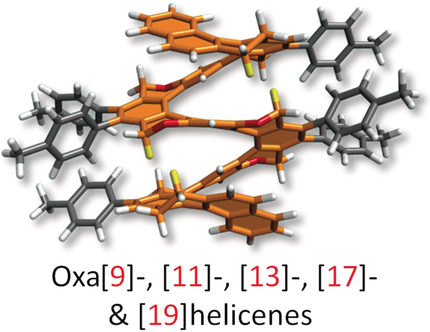
Think big: Oxahelicenes comprising up to 19 fused rings (see example) were synthesized by multiple cobalt(I)-mediated cycloisomerization reactions of oligoynes, most efficiently in a flow reactor. The stereogenic centers in enantiomerically pure substrates steered the diastereoselective polycyclization of the oligoynes. Single-molecule conductivity was studied in a pyridooxa[9]helicene by the break-junction method.
Abstract
A series of oxahelicenes composed of ortho/meta-annulated benzene/pyridine and 2H-pyran rings were synthesized on the basis of the cobalt(I)-mediated (or rhodium(I)- or nickel(0)-mediated) double, triple, or quadruple [2+2+2] cycloisomerization of branched aromatic hexa-, nona-, or dodecaynes, thus allowing the construction of 6, 9, or 12 rings in a single operation. The use of a flow reactor was found to be beneficial for the multicyclization reactions. The stereogenic centers present in some of the oligoynes steered the helical folding in such a way that the final oxa[9]-, [13]-, [17]- and [19]helicenes were obtained in both enantiomerically and diastereomerically pure form. Specifically, the oxa[19]helicenes beat the current record in the length of a helicene backbone. Single-molecule conductivity was studied by the mechanically controllable break-junction method with a pyridooxa[9]helicene.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201700341-sup-0001-misc_information.pdf12.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C.-F. Chen, Y. Shen, Helicene Chemistry: From Synthesis to Applications, Springer, Berlin, 2017.
10.1007/978-3-662-53168-6 Google Scholar
- 2
- 2aX. Quan, C. W. Marvin, L. Seebald, G. R. Hutchison, J. Phys. Chem. C 2013, 117, 16783–16790;
- 2bL. Rulíšek, O. Exner, L. Cwiklik, P. Jungwirth, I. Starý, L. Pospíšil, Z. Havlas, J. Phys. Chem. C 2007, 111, 14948–14955;
- 2cP. Rempala, B. T. King, J. Chem. Theory Comput. 2006, 2, 1112–1118.
- 3
- 3aJ. Vacek, J. Vacek Chocholoušová, I. G. Stará, I. Starý, Y. Dubi, Nanoscale 2015, 7, 8793–8802;
- 3bJ. Storch, J. Zadny, T. Strasak, M. Kubala, J. Sykora, M. Dusek, V. Cirkva, P. Matejka, M. Krbal, J. Vacek, Chem. Eur. J. 2015, 21, 2343–2347;
- 3cX. Gu, X. Xu, H. Li, Z. Liu, Q. Miao, J. Am. Chem. Soc. 2015, 137, 16203–16208;
- 3dH. Hirai, K. Nakajima, S. Nakatsuka, K. Shiren, J. Ni, S. Nomura, T. Ikuta, T. Hatakeyama, Angew. Chem. Int. Ed. 2015, 54, 13581–13585; Angew. Chem. 2015, 127, 13785–13789;
- 3eY. Yang, R. C. da Costa, M. J. Fuchter, A. J. Campbell, Nat. Photonics 2013, 7, 634–638.
- 4
- 4aN. Saleh, M. Srebro, T. Reynaldo, N. Vanthuyne, L. Toupet, V. Y. Chang, G. Muller, J. A. G. Williams, C. Roussel, J. Autschbach, et al., Chem. Commun. 2015, 51, 3754–3757;
- 4bL. Pospíšil, L. Bednárová, P. Štěpánek, P. Slavíček, J. Vávra, M. Hromadová, H. Dlouhá, J. Tarábek, F. Teplý, J. Am. Chem. Soc. 2014, 136, 10826–10829;
- 4cD. Schweinfurth, M. Zalibera, M. Kathan, C. Shen, M. Mazzolini, N. Trapp, J. Crassous, G. Gescheidt, F. Diederich, J. Am. Chem. Soc. 2014, 136, 13045–13052;
- 4dR. Hassey, E. J. Swain, N. I. Hammer, D. Venkataraman, M. D. Barnes, Science 2006, 314, 1437–1439;
- 4eT. Verbiest, S. V. Elshocht, M. Kauranen, L. Hellemans, J. Snauwaert, C. Nuckolls, T. J. Katz, A. Persoons, Science 1998, 282, 913–915.
- 5K. Mori, T. Murase, M. Fujita, Angew. Chem. Int. Ed. 2015, 54, 6847–6851; Angew. Chem. 2015, 127, 6951–6955.
- 6S. Han, D. R. Anderson, A. D. Bond, H. V. Chu, R. L. Disch, D. Holmes, J. M. Schulman, S. J. Teat, K. P. C. Vollhardt, G. D. Whitener, Angew. Chem. Int. Ed. 2002, 41, 3227–3230;
10.1002/1521-3773(20020902)41:17<3227::AID-ANIE3227>3.0.CO;2-T CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 3361–3364.
- 7
- 7aD. Z. Wang, T. J. Katz, J. Golen, A. L. Rheingold, J. Org. Chem. 2004, 69, 7769–7771;
- 7bJ. Roose, S. Achermann, O. Dumele, F. Diederich, Eur. J. Org. Chem. 2013, 3223–3231.
- 8K. Yamada, S. Ogashiwa, H. Tanaka, H. Nakagawa, H. Kawazura, Chem. Lett. 1981, 10, 343–346.
- 9R. H. Martin, M. Baes, Tetrahedron 1975, 31, 2135–2137.
- 10R. H. Martin, G. Morren, J. J. Schurter, Tetrahedron Lett. 1969, 10, 3683–3688.
10.1016/S0040-4039(01)88487-2 Google Scholar
- 11B. Milde, M. Leibeling, M. Pawliczek, J. Grunenberg, P. G. Jones, D. B. Werz, Angew. Chem. Int. Ed. 2015, 54, 1331–1335; Angew. Chem. 2015, 127, 1347–1351.
- 12H. Wynberg, M. Groen, H. Schadenberg, J. Org. Chem. 1971, 36, 2797–2809.
- 13M. Miyasaka, A. Rajca, M. Pink, S. Rajca, J. Am. Chem. Soc. 2005, 127, 13806–13807.
- 14Y. Kimura, N. Fukawa, Y. Miyauchi, K. Noguchi, K. Tanaka, Angew. Chem. Int. Ed. 2014, 53, 8480–8483; Angew. Chem. 2014, 126, 8620–8623.
- 15P. Sehnal, I. G. Stará, D. Šaman, M. Tichý, J. Míšek, J. Cvačka, L. Rulíšek, J. Chocholoušová, J. Vacek, G. Goryl, M. Szymonski, I. Císařová, I. Starý, Proc. Natl. Acad. Sci. USA 2009, 106, 13169–13174.
- 16H. Wynberg, M. B. Groen, J. Am. Chem. Soc. 1970, 92, 6664–6665.
- 17D. J. Hill, M. J. Mio, R. B. Prince, T. S. Hughes, J. S. Moore, Chem. Rev. 2001, 101, 3893–4012.
- 18R. W. Hoffmann, Chem. Rev. 1989, 89, 1841–1860.
- 19
- 19aM. Šámal, S. Chercheja, J. Rybáček, J. Vacek Chocholoušová, J. Vacek, L. Bednárová, D. Šaman, I. G. Stará, I. Starý, J. Am. Chem. Soc. 2015, 137, 8469–8474;
- 19bJ. Žádný, A. Jančařík, A. Andronova, M. Šámal, J. Vacek Chocholoušová, J. Vacek, R. Pohl, D. Šaman, I. Císařová, I. G. Stará, I. Starý, Angew. Chem. Int. Ed. 2012, 51, 5857–5861; Angew. Chem. 2012, 124, 5959–5963.
- 20
- 20aM. S. Newman, R. S. Darlak, L. Tsai, J. Am. Chem. Soc. 1967, 89, 6191–6193;
- 20bC. Goedicke, H. Stegemeyer, Tetrahedron Lett. 1970, 11, 937–940;
10.1016/S0040-4039(01)97871-2 Google Scholar
- 20cR. H. Martin, M. J. Marchant, Tetrahedron 1974, 30, 343–345.
- 21
- 21aM. Buchta, J. Rybáček, A. Jančařík, A. A. Kudale, M. Buděšínský, J. V. Chocholoušová, J. Vacek, L. Bednárová, I. Císařová, G. J. Bodwell, I. Starý, I. G. Stará, Chem. Eur. J. 2015, 21, 8910–8917;
- 21bA. Jančařík, J. Rybáček, K. Cocq, J. Vacek Chocholoušová, J. Vacek, R. Pohl, L. Bednárová, P. Fiedler, I. Císařová, I. G. Stará, I. Starý, Angew. Chem. Int. Ed. 2013, 52, 9970–9975; Angew. Chem. 2013, 125, 10154–10159.
- 22D. Xiang, H. Jeong, T. Lee, D. Mayer, Adv. Mater. 2013, 25, 4845–4867.
- 23
- 23aY.-D. Guo, X.-H. Yan, Y. Xiao, C.-S. Liu, Sci. Rep. 2015, 5, 16731;
- 23bL. Chen, Y.-H. Wang, B. He, H. Nie, R. Hu, F. Huang, A. Qin, X.-S. Zhou, Z. Zhao, B. Z. Tang, Angew. Chem. Int. Ed. 2015, 54, 4231–4235; Angew. Chem. 2015, 127, 4305–4309;
- 23cG. Treboux, P. Lapstun, Z. H. Wu, K. Silverbrook, Chem. Phys. Lett. 1999, 301, 493–497.
- 24
- 24aV. Kiran, S. P. Mathew, S. R. Cohen, I. Hernández Delgado, J. Lacour, R. Naaman, Adv. Mater. 2016, 28, 1957–1962;
- 24bB. Gohler, V. Hamelbeck, T. Z. Markus, M. Kettner, G. F. Hanne, Z. Vager, R. Naaman, H. Zacharias, Science 2011, 331, 894–897.
- 25G. C. Solomon, C. Herrmann, J. Vura-Weis, M. R. Wasielewski, M. A. Ratner, J. Am. Chem. Soc. 2010, 132, 7887–7889.