Total Syntheses of Sarcandrolide J and Shizukaol D: Lindenane Sesquiterpenoid [4+2] Dimers
Correction(s) for this article
-
Corrigendum: Total Syntheses of Sarcandrolide J and Shizukaol D: Lindenane Sesquiterpenoid [4+2] Dimers
- Volume 61Issue 2Angewandte Chemie International Edition
- First Published online: January 4, 2022
Changchun Yuan
Key Laboratory of Green Chemistry & Technology of the Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
These authors contributed equally to this work.
Search for more papers by this authorBiao Du
Key Laboratory of Green Chemistry & Technology of the Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
These authors contributed equally to this work.
Search for more papers by this authorHeping Deng
Key Laboratory of Green Chemistry & Technology of the Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
Search for more papers by this authorYi Man
Key Laboratory of Green Chemistry & Technology of the Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Bo Liu
Key Laboratory of Green Chemistry & Technology of the Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, 210009 China
Search for more papers by this authorChangchun Yuan
Key Laboratory of Green Chemistry & Technology of the Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
These authors contributed equally to this work.
Search for more papers by this authorBiao Du
Key Laboratory of Green Chemistry & Technology of the Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
These authors contributed equally to this work.
Search for more papers by this authorHeping Deng
Key Laboratory of Green Chemistry & Technology of the Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
Search for more papers by this authorYi Man
Key Laboratory of Green Chemistry & Technology of the Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Bo Liu
Key Laboratory of Green Chemistry & Technology of the Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Rd., Chengdu, Sichuan, 610064 China
State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, 210009 China
Search for more papers by this authorGraphical Abstract
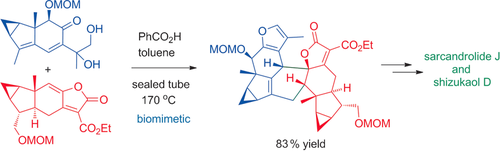
Taking up the challenge: Total syntheses of the title compounds were achieved on the basis of a modified biosynthetic pathway. A conjugated furanyl diene was generated in situ for the first time, and a cascade featuring furan formation/alkene isomerization/Diels–Alder cycloaddition was devised to construct the congested polycyclic architecture of the target molecules.
Abstract
The syntheses of members of a family of lindenane sesquiterpenoid [4+2] dimers led to the total syntheses of sarcandrolide J and shizukaol D. Inspired by a modified biosynthetic pathway, a cascade featuring furan formation/alkene isomerization/Diels–Alder cycloaddition was devised to construct the congested polycyclic architecture of the target molecules with the correct stereochemistry. This study presents a pioneering synthetic entry to this family of natural products and paves the way for fully exploring their biological functions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201610484-sup-0001-misc_information.pdf8.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. Kawabata, Y. Fukushi, S. Tahara, J. Mizutani, Phytochemistry 1990, 29, 2332–2334.
- 2For seminal review articles, see:
- 2aC.-M. Cao, Y. Peng, Q.-W. Shi, P.-G. Xiao, Chem. Biodiversity 2008, 5, 219–238;
- 2bY.-J. Xu, Chem. Biodiversity 2013, 10, 1754–1773;
- 2cA.-R. Wang, H.-C. Song, H.-M. An, Q. Huang, X. Luo, J.-Y. Dong, Chem. Biodiversity 2015, 12, 451–473.
- 3For recent isolation of [4+2] dimeric sesquiterpenoids in this family, see:
- 3aG. Ni, H. Zhang, H.-C. Liu, S.-P. Yang, M.-Y. Geng, J.-M. Yue, Tetrahedron 2013, 69, 564–569;
- 3bL.-J. Wang, J. Xiong, S.-T. Liu, X.-H. Liu, H.-F. Hu, Chem. Biodiversity 2014, 11, 919–928;
- 3cP. Wang, J. Luo, Y.-M. Zhang, L.-Y. Kong, Tetrahedron 2015, 71, 5362–5370;
- 3dL.-J. Wang, J. Xiong, C. Lau, L.-L. Pan, J.-F. Hu, J. Asian Nat. Prod. Res. 2015, 17, 1220–1230;
- 3eX.-W. Shi, Q.-Q. Lu, G. Pescitelli, T. Ivšić, J.-H. Zhou, J.-M. Gao, Chirality 2016, 28, 158–163;
- 3fY.-Q. Guo, J.-J. Zhao, Z.-Z. Li, G.-H. Tang, Z.-M. Zhao, S. Yin, Bioorg. Med. Chem. Lett. 2016, 26, 3163–3166.
- 4S.-P. Yang, Z.-B. Gao, F.-D. Wang, S.-G. Liao, H.-D. Chen, C.-R. Zhang, G.-Y. Hu, J.-M. Yue, Org. Lett. 2007, 9, 903–906.
- 5J. Kawabata, J. Mizutani, Phytochemistry 1992, 31, 1293–1296.
- 6
- 6aS. W. Kwon, Y. K. Kim, J. Y. Kim, H. S. Ryu, H. K. Lee, J. S. Kang, H. M. Kim, J. T. Hong, Y. Kim, S.-B. Han, Biomol. Ther. 2011, 19, 59–64;
- 6bO. E. Kwon, H. S. Lee, S. W. Lee, K. Bae, K. Kim, M. Hayashi, M.-C. Rho, Y.-K. Kim, J. Ethnopharmacol. 2006, 104, 270–277;
- 6cP.-L. Fang, Y.-L. Cao, H. Yan, L.-L. Pan, S.-C. Liu, N.-B. Gong, Y. Lü, C.-X. Chen, H.-M. Zhong, Y. Guo, H.-Y. Liu, J. Nat. Prod. 2011, 74, 1408–1413.
- 7
- 7aR. Hu, H. Yan, X. Hao, H. Liu, J. Wu, PLoS ONE 2013, 8, e 73527;
- 7bL. Tang, H. Zhu, X. Yang, F. Xie, J. Peng, D. Jiang, J. Xie, M. Qi, L. Yu, PLoS ONE 2015, 11, e 0152012.
- 8
- 8aT. W. Fenton, D. Schwaebisch, A. V. W. Mayweg, V. Lee, R. M. Adlington, J. E. Baldwin, Synlett 2007, 2679–2682;
- 8bT. W. Fenlon, M. W. Jones, R. M. Adlington, V. Lee, Org. Biomol. Chem. 2013, 11, 8026–8029;
- 8cY. Liu, F.-J. Nan, Tetrahedron Lett. 2010, 51, 1374–1376;
- 8dH. Zhang, F. Nan, Chin. J. Chem. 2013, 31, 84–92;
- 8eS. Qian, G. Zhao, Synlett 2011, 722–724;
- 8fS. Qian, G. Zhao, Chem. Commun. 2012, 48, 3530–3532;
- 8gS. Qian, G. Zhao, Tetrahedron 2013, 69, 11169–11173;
- 8hG. Yue, L. Yang, C. Yuan, X. Jiang, B. Liu, Org. Lett. 2011, 13, 5406–5408;
- 8iG. Yue, L. Yang, C. Yuan, B. Du, B. Liu, Tetrahedron 2012, 68, 9624–9637;
- 8jS. Ramesh, G. Mehta, Tetrahedron Lett. 2015, 56, 3941–3944.
- 9
- 9aY.-S. Lu, X.-S. Peng, Org. Lett. 2011, 13, 2940–2943; correction: Y.-S. Lu, X.-S. Peng, Org. Lett. 2016, 18, 5447–5448;
- 9bL. Yang, G. Yue, C. Yuan, B. Du, H. Deng, B. Liu, Synlett 2014, 2471–2474.
- 10M. Uchida, G. Kusano, Y. Kondo, S. Nozoe, Heterocycles 1978, 9, 139–144.
- 11
- 11aJ. Kawabata, S. Tahara, J. Mizutani, A. Furusaki, N. Hashiba, T. Matsumoto, Agric. Biol. Chem. 1979, 43, 885–887;
- 11bJ. Kawabata, S. Tahara, J. Mizutani, Agric. Biol. Chem. 1981, 45, 1447–1453.
- 12See the Supporting Information for details.
- 13
- 13aC. Yuan, B. Du, L. Yang, B. Liu, J. Am. Chem. Soc. 2013, 135, 9291–9294;
- 13bB. Du, C. Yuan, T. Yu, L. Yang, B. Liu, S. Qin, Chem. Eur. J. 2014, 20, 2613–2622.
- 14
- 14aK. Takeda, H. Ishii, T. Tozyo, H. Minato, J. Chem. Soc. C 1969, 1920–1921;
- 14bK. Takeda, M. Ikuta, M. Miyawaki, Tetrahedron 1964, 20, 2991–2997;
- 14cH. Ishii, T. Tozyo, M. Nakamura, K. Takeda, Tetrahedron 1968, 24, 625–631.
- 15
- 15aY. Yang, J. Li, B. Du, C. Yuan, B. Liu, S. Qin, Chem. Commun. 2015, 51, 6179–6182;
- 15bSee Supporting Information for details.
- 16Y. Ito, T. Hirao, T. Saegusa, J. Org. Chem. 1978, 43, 1011–1013.
- 17A. R. Angeles, S. P. Waters, S. J. Danishefsky, J. Am. Chem. Soc. 2008, 130, 13765–13770.
- 18For a recent review article on the application of Diels–Alder reactions in total synthesis, see: E. Chirkin, F.-H. Porée, Curr. Org. Chem. 2016, 20, 2284–2325, and references therein.
- 19S. Bur, A. Padwa in Methods and Applications of Cycloaddition Reactions in Organic Synthesis (Eds.: ), Wiley-VCH, Hoboken, 2014, pp. 355–406.
10.1002/9781118778173.ch13 Google Scholar
- 20
- 20aC. L. Hugelshofer, T. Magaue, J. Am. Chem. Soc. 2015, 137, 3807–3810;
- 20bN. Kornblum, H. E. DeLaMare, J. Am. Chem. Soc. 1951, 73, 880–881.