Generation of an 4-Isoxazolyl Anion Species: Facile Access to Multifunctionalized Isoxazoles
Graphical Abstract
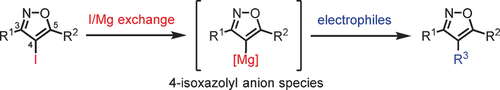
Taking position: Preparation of a 4-isoxazolyl anion species from 4-iodoisoxazole using iPrMgCl⋅LiCl enabled introduction of a wide variety of functional groups into the 4-position of the isoxazole ring in good to excellent yields. This approach provides various isoxazolyl metal species which can be used for multifunctionalization of isoxazoles. The step-by-step synthesis of 3,4,5-trisubstituted isoxazoles was achieved by using the this 4-isoxazolyl anion method.
Abstract
A direct functionalization of unsubstituted isoxazole (1) was achieved by generation of 4-isoxazolyl anion species (3). An efficient 4-iodination of isoxazole and halogen–metal exchange reaction using a turbo Grignard reagent (iPrMgCl⋅ LiCl) were essential for the generation of 3, which reacted with various electrophiles to give 4-functionalized isoxazoles in good to high yields. Isoxazolyl boronate, boronic acid, and stannane were also synthesized as useful building blocks from 1. The current methods enabled us to synthesize multi-functionalized isoxazoles by introducing each substituent into the desired positions. Furthermore, total synthesis of triumferol, which was isolated from Triumfetta rhomboidea, was achieved from 1 in only three steps.