Mediating Order and Modulating Porosity by Controlled Hydrolysis in a Phosphonate Monoester Metal–Organic Framework
Benjamin S. Gelfand
Department of Chemistry, University of Calgary, 2500 University Drive NW, Calgary, AB, T2N 1N4 Canada
Search for more papers by this authorRacheal P. S. Huynh
Department of Chemistry, University of Calgary, 2500 University Drive NW, Calgary, AB, T2N 1N4 Canada
Search for more papers by this authorRoger K. Mah
Department of Chemistry, University of Calgary, 2500 University Drive NW, Calgary, AB, T2N 1N4 Canada
Search for more papers by this authorCorresponding Author
Prof. George K. H. Shimizu
Department of Chemistry, University of Calgary, 2500 University Drive NW, Calgary, AB, T2N 1N4 Canada
Search for more papers by this authorBenjamin S. Gelfand
Department of Chemistry, University of Calgary, 2500 University Drive NW, Calgary, AB, T2N 1N4 Canada
Search for more papers by this authorRacheal P. S. Huynh
Department of Chemistry, University of Calgary, 2500 University Drive NW, Calgary, AB, T2N 1N4 Canada
Search for more papers by this authorRoger K. Mah
Department of Chemistry, University of Calgary, 2500 University Drive NW, Calgary, AB, T2N 1N4 Canada
Search for more papers by this authorCorresponding Author
Prof. George K. H. Shimizu
Department of Chemistry, University of Calgary, 2500 University Drive NW, Calgary, AB, T2N 1N4 Canada
Search for more papers by this authorGraphical Abstract
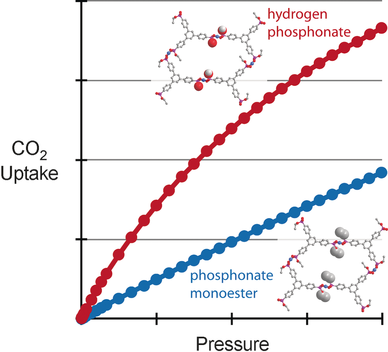
Fine tuning of porosity: Controlled in situ ester hydrolysis during metal complexation enables the formation of isostructural phosphonate monoester and phosphonate MOFs with very different gas sorption properties. By tuning of the synthetic conditions, it is possible to selectively remove some of the monoesters lining the pore to form a hydrogen phosphonate with increased affinity for CO2.
Abstract
A crystalline and permanently porous copper phosphonate monoester framework has been synthesized from a tetraaryl trigonal phosphonate monoester linker. This material has a surface area over 1000 m2 g−1, as measured by N2 sorption, the highest reported for a phosphonate-based metal–organic framework (MOF). The monoesters result in hydrophobic pore surfaces that give a low heat of adsorption for CO2 and low calculated selectivity for CO2 over N2 and CH4 in binary mixtures. By careful manipulation of synthetic conditions, it is possible to selectively remove some of the monoesters lining the pore to form a hydrogen phosphonate while giving an isomorphous structure. This increases the affinity of the framework for CO2 giving higher ambient uptake, higher heat of adsorption, and much higher calculated selectivity for CO2 over both N2 and CH4. Formation of the acid groups is noteworthy as complexation with the parent acid gives a different structure.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201607745-sup-0001-misc_information.pdf1.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. Quadrelli, S. Peterson, Energy Policy 2007, 35, 5938.
- 2E. J. Granite, H. W. Pennline, Ind. Eng. Chem. Res. 2002, 41, 5470.
- 3L. Deng, M. B. Hägg, Int. J. Greenhouse Gas Control 2010, 4, 638.
- 4
- 4aT. Rodenas, I. Luz, G. Prieto, B. Seoane, H. Miro, A. Corma, F. Kapteijn, F. X. Llabrés i Xamena, J. Gascon, Nat. Mater. 2014, 14, 48;
- 4bS. S. Nagarkar, A. K. Chaudhari, S. K. Ghosh, Inorg. Chem. 2012, 51, 572;
- 4cP. S. Bárcia, L. Bastin, E. J. Hurtado, J. A. C. Silva, A. E. Rodrigues, B. Chen, Sep. Sci. Technol. 2008, 43, 3494;
- 4dJ.-R. Li, Y. Ma, M. C. McCarthy, J. Sculley, J. Yu, H.-K. Jeong, P. B. Balbuena, H.-C. Zhou, Coord. Chem. Rev. 2011, 255, 1791.
- 5
- 5aR. K. Mah, B. S. Gelfand, J. M. Taylor, G. K. H. Shimizu, Inorg. Chem. Front. 2015, 2, 273;
- 5bB. S. Gelfand, J. Lin, G. K. H. Shimizu, Inorg. Chem. 2015, 54, 1185;
- 5cJ. M. Taylor, R. Vaidhyanathan, S. S. Iremonger, G. K. H. Shimizu, J. Am. Chem. Soc. 2012, 134, 14338;
- 5dJ.-W. Zhang, C.-C. Zhao, Y.-P. Zhao, H.-Q. Xu, Z.-Y. Du, H.-L. Jiang, CrystEngComm 2014, 16, 6635.
- 6
- 6aS. Pili, S. P. Argent, C. G. Morris, P. Rought, V. Garc, I. P. Silverwood, T. L. Easun, M. Li, M. R. Warren, C. A. Murray, C. C. Tang, S. Yang, M. Schröder, J. Am. Chem. Soc. 2016, 138, 6352;
- 6bK. J. Gagnon, H. P. Perry, A. Clearfield, Chem. Rev. 2012, 112, 1034;
- 6cM. Taddei, F. Costantino, R. Vivani, Eur. J. Inorg. Chem. 2016, 1;
- 6dJ. Goura, V. Chandrasekhar, Chem. Rev. 2015, 115, 6854;
- 6eR. Vaidhyanathan, A. H. Mahmoudkhani, G. K. H. Shimizu, Can. J. Chem. 2009, 87, 247;
- 6fA. U. Ortiz, A. Boutin, K. J. Gagnon, A. Clearfield, F.-X. Coudert, J. Am. Chem. Soc. 2014, 136, 11540;
- 6gN. Hermer, N. Stock, Dalton Trans. 2015, 44, 3720;
- 6hP. L. Llewellyn, M. Garcia-Rates, L. Gaberová, S. R. Miller, T. Devic, J.-C. Lavalley, S. Bourrelly, E. Bloch, Y. Filinchuk, P. A. Wright, et al., J. Phys. Chem. C 2015, 119, 4208;
- 6iK. Maeda, F. Mizukami, Angew. Chem. Int. Ed. Engl. 1994, 33, 2335; Angew. Chem. 1994, 106, 2427;
- 6jR. Howlader, M. G. Walawalkar, R. Murugavel, Inorg. Chim. Acta 2013, 405, 147;
- 6kM. Taddei, F. Costantino, F. Marmottini, A. Comotti, P. Sozzani, R. Vivani, Chem. Commun. 2014, 50, 14831;
- 6lA. Kondo, T. Satomi, K. Azuma, R. Takeda, K. Maeda, Dalton Trans. 2015, 44, 12717;
- 6mS. Hossain, S. K. Gupta, R. Murugavel, CrystEngComm 2015, 17, 4355.
- 7S. S. Iremonger, J. Liang, R. Vaidhyanathan, I. Martens, G. K. H. Shimizu, T. D. Daff, M. Z. Aghaji, S. Yeganegi, T. K. Woo, J. Am. Chem. Soc. 2011, 133, 20048.
- 8Structure data for CALF-33-Et2H (see SI for details): C56H52Cu3O18P6 (Mw=1389.45), T=173(2) K, triclinic, space group
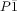 (No. 2), a=5.08130(10), b=15.1585(3), c=29.3246(7) Å, α=83.8820(10), β=85.7280(10), γ=82.705(2)°, V=2223.42(8) Å3, Z=1, ρcalcd=1.036 g cm−3, μ=0.866 mm−1, measured reflections, 7376 unique reflections (Rint=0.0939), R1=0.0700 for 3758 observed reflections (I>2σ(I)) and 379 parameters (after accounting for disordered solvent with the SQUEEZE function in Platon[21]). CCDC 1496410 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
(No. 2), a=5.08130(10), b=15.1585(3), c=29.3246(7) Å, α=83.8820(10), β=85.7280(10), γ=82.705(2)°, V=2223.42(8) Å3, Z=1, ρcalcd=1.036 g cm−3, μ=0.866 mm−1, measured reflections, 7376 unique reflections (Rint=0.0939), R1=0.0700 for 3758 observed reflections (I>2σ(I)) and 379 parameters (after accounting for disordered solvent with the SQUEEZE function in Platon[21]). CCDC 1496410 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 9Accelrys Software Inc., 2011.
- 10T. Yamada, H. Kitagawa, CrystEngComm 2012, 14, 4148.
- 11
- 11aK. Wang, D. Feng, T.-F. Liu, J. Su, S. Yuan, Y.-P. Chen, M. Bosch, X. Zou, H.-C. Zhou, J. Am. Chem. Soc. 2014, 136, 13983;
- 11bP. D. C. Dietzel, B. Panella, M. Hirscher, R. Blom, H. Fjellvåg, Chem. Commun. 2006, 959.
- 12
- 12aB. Mu, P. M. Schoenecker, K. S. Walton, J. Phys. Chem. C 2010, 114, 6464;
- 12bP. S. Bárcia, L. Bastin, E. J. Hurtado, J. A. C. Silva, A. E. Rodrigues, B. Chen, Sep. Sci. Technol. 2008, 43, 3494;
- 12cL. Bastin, P. S. Bárcia, E. J. Hurtado, J. A. C. Silva, A. E. Rodrigues, B. Chen, J. Phys. Chem. C 2008, 112, 1575.
- 13R. Vaidhyanathan, S. S. Iremonger, G. K. H. Shimizu, P. G. Boyd, S. Alavi, T. K. Woo, Science 2010, 330, 650.
- 14
- 14aZ. Chen, S. Xiang, H. D. Arman, J. U. Mondal, P. Li, D. Zhao, B. Chen, Inorg. Chem. 2011, 50, 3442;
- 14bM. Du, C.-P. Li, M. Chen, Z.-W. Ge, X. Wang, L. Wang, C.-S. Liu, J. Am. Chem. Soc. 2014, 136, 10906.
- 15
- 15aI. A. Ibarra, A. Mace, S. Yang, J. Sun, S. Lee, J.-S. Chang, A. Laaksonen, M. Schröder, X. Zou, Inorg. Chem. 2016, 55, 7219;
- 15bK. Jayaramulu, S. K. Reddy, A. Hazra, S. Balasubramanian, T. K. Maji, Inorg. Chem. 2012, 51, 7103.
- 16
- 16aC. M. Simon, B. Smit, M. Haranczyk, Comput. Phys. Commun. 2015, 1;
- 16bA. L. Myers, J. M. Prausnitz, AIChE J. 1965, 11, 121.
- 17
- 17aM. T. Wharmby, G. M. Pearce, J. P. S. Mowat, J. M. Griffin, S. E. Ashbrook, P. A. Wright, L.-H. Schilling, A. Lieb, N. Stock, S. Chavan, et al., Microporous Mesoporous Mater. 2012, 157, 3;
- 17bJ. M. Simmons, H. Wu, W. Zhou, T. Yildirim, Energy Environ. Sci. 2011, 4, 2177.
- 18A. Ö. Yazaydın, R. Q. Snurr, T.-H. Park, K. Koh, J. Liu, M. D. Levan, A. I. Benin, P. Jakubczak, M. Lanuza, D. B. Galloway, et al., J. Am. Chem. Soc. 2009, 131, 18198.
- 19
- 19aM. Kontturi, E. Laurila, R. Mattsson, S. Peräniemi, J. J. Vepsäläinen, M. Ahlgrén, Inorg. Chem. 2005, 44, 2400;
- 19bJ. Jokiniemi, J. J. Vepsäläinen, H. Nätkinniemi, S. Peräniemi, M. Ahlgrén, CrystEngComm 2009, 11, 2431.
- 20P. Ayyappan, O. R. Evans, Y. Cui, K. A. Wheeler, W. Lin, Inorg. Chem. 2002, 41, 4978.
- 21A. L. Spek, PLATON, A Multipurpose Crystallographic Tool, Utrecht University, Utrecht, The Netherlands, 2007.