Mixed Directing-Group Strategy: Oxidative C−H/C−H Bond Arylation of Unactivated Arenes by Cobalt Catalysis
Cong Du
College of Chemistry and Molecular Engineering, Zhengzhou University, Kexue avenue 100, Zhengzhou, P.R. China
These authors contributed equally to this work.
Search for more papers by this authorPeng-Xiang Li
College of Chemistry and Molecular Engineering, Zhengzhou University, Kexue avenue 100, Zhengzhou, P.R. China
These authors contributed equally to this work.
Search for more papers by this authorDr. Xinju Zhu
College of Chemistry and Molecular Engineering, Zhengzhou University, Kexue avenue 100, Zhengzhou, P.R. China
Search for more papers by this authorJian-Feng Suo
College of Chemistry and Molecular Engineering, Zhengzhou University, Kexue avenue 100, Zhengzhou, P.R. China
Search for more papers by this authorCorresponding Author
Dr. Jun-Long Niu
College of Chemistry and Molecular Engineering, Zhengzhou University, Kexue avenue 100, Zhengzhou, P.R. China
Search for more papers by this authorCorresponding Author
Prof. Mao-Ping Song
College of Chemistry and Molecular Engineering, Zhengzhou University, Kexue avenue 100, Zhengzhou, P.R. China
Search for more papers by this authorCong Du
College of Chemistry and Molecular Engineering, Zhengzhou University, Kexue avenue 100, Zhengzhou, P.R. China
These authors contributed equally to this work.
Search for more papers by this authorPeng-Xiang Li
College of Chemistry and Molecular Engineering, Zhengzhou University, Kexue avenue 100, Zhengzhou, P.R. China
These authors contributed equally to this work.
Search for more papers by this authorDr. Xinju Zhu
College of Chemistry and Molecular Engineering, Zhengzhou University, Kexue avenue 100, Zhengzhou, P.R. China
Search for more papers by this authorJian-Feng Suo
College of Chemistry and Molecular Engineering, Zhengzhou University, Kexue avenue 100, Zhengzhou, P.R. China
Search for more papers by this authorCorresponding Author
Dr. Jun-Long Niu
College of Chemistry and Molecular Engineering, Zhengzhou University, Kexue avenue 100, Zhengzhou, P.R. China
Search for more papers by this authorCorresponding Author
Prof. Mao-Ping Song
College of Chemistry and Molecular Engineering, Zhengzhou University, Kexue avenue 100, Zhengzhou, P.R. China
Search for more papers by this authorGraphical Abstract
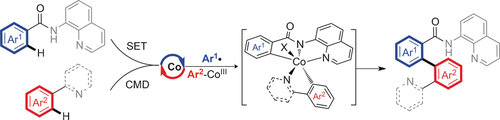
[Co(acac)3]-catalyzed oxidative C−H/C−H bond arylation of unactivated arenes enables the transformation of a wide range of benzamides and arylpyridines to afford bifunctionalized biaryls. A single-electron transfer (SET) and a concerted metalation–deprotonation (CMD) are involved. Moreover, the aryl radicals were trapped by 2,6-di-tert-butyl-4-methylphenol to form benzylated products.
Abstract
A mixed directing-group strategy for inexpensive [Co(acac)3]-catalyzed oxidative C−H/C−H bond arylation of unactivated arenes has been disclosed. This strategy enables the arylation of a wide range of benzamide and arylpyridines effectively to afford novel bifunctionalized biaryls, which are difficult to achieve by common synthetic routes. Two different pathways, namely, a single-electron-transmetalation process (8-aminoquinoline-directed) and a concerted metalation–deprotonation process (pyridine-directed), were involved to activate two different inert aromatic C−H bonds. Moreover, the aryl radicals have been trapped by 2,6-di-tert-butyl-4-methylphenol to form benzylated products. This unique strategy should be useful in the design of other arene C−H/C−H cross-couplings as well.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201607719-sup-0001-misc_information.pdf3.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews on aryl–aryl bond formation, see:
- 1aG. P. McGlacken, L. M. Bateman, Chem. Soc. Rev. 2009, 38, 2447–2464;
- 1bI. Hussain, T. Singh, Adv. Synth. Catal. 2014, 356, 1661–1696.
- 2For selected reviews on CDC reactions, see:
- 2aC.-J. Li, Acc. Chem. Res. 2009, 42, 335–344;
- 2bC. S. Yeung, V. M. Dong, Chem. Rev. 2011, 111, 1215–1292.
- 3For selected examples, see:
- 3aK. L. Hull, M. S. Sanford, J. Am. Chem. Soc. 2007, 129, 11904–11905;
- 3bB.-J. Li, S.-L. Tian, Z. Fang, Z.-J. Shi, Angew. Chem. Int. Ed. 2008, 47, 1115–1118; Angew. Chem. 2008, 120, 1131–1134;
- 3cY. Wei, W. Su, J. Am. Chem. Soc. 2010, 132, 16377–16379;
- 3dX. Wang, D. Leow, J.-Q. Yu, J. Am. Chem. Soc. 2011, 133, 13864–13867;
- 3eJ. Karthikeyan, C.-H. Cheng, Angew. Chem. Int. Ed. 2011, 50, 9880–9883; Angew. Chem. 2011, 123, 10054–10057;
- 3fD. Sun, B. Li, J. Lan, Q. Huang, J. You, Chem. Commun. 2016, 52, 3635–3638;
- 3gS.-J. Lou, Y.-J. Mao, D.-Q. Xu, J.-Q. He, Q. Chen, Z.-Y. Xu, ACS Catal. 2016, 6, 3890–3894.
- 4
- 4aJ. Wencel-Delord, C. Nimphius, F. W. Patureau, F. Glorius, Angew. Chem. Int. Ed. 2012, 51, 2247–2251; Angew. Chem. 2012, 124, 2290–2294;
- 4bX.-S. Zhang, Y.-F. Zhang, Z.-W. Li, F.-X. Luo, Z.-J. Shi, Angew. Chem. Int. Ed. 2015, 54, 5478–5482; Angew. Chem. 2015, 127, 5568–5572;
- 4cK. Matsumoto, M. Yoshida, M. Shindo, Angew. Chem. Int. Ed. 2016, 55, 5272–5276; Angew. Chem. 2016, 128, 5358–5362.
- 5For recent reviews, see:
- 5aM. Moselage, J. Li, L. Ackermann, ACS Catal. 2016, 6, 498–525;
- 5bD. Wei, X. Zhu, J.-L. Niu, M.-P. Song, ChemCatChem 2016, 8, 1242–1263.
- 6For recent reviews on the low-valent cobalt-catalyzed C−H bond activation, see:
- 6aL. Ackermann, J. Org. Chem. 2014, 79, 8948–8954;
- 6bK. Gao, N. Yoshikai, Acc. Chem. Res. 2014, 47, 1208–1219.
- 7Cobalt-promoted dimerization of arenes:
- 7aL. Grigorjeva, O. Daugulis, Org. Lett. 2015, 17, 1204–1207;
- 7bY. Xie, D. Xu, W.-W. Sun, S.-J. Zhang, X.-P. Dong, B. Liu, Y. Zhou, B. Wu, Asian J. Org. Chem. 2016, 5, 961–965.
- 8For selected examples of Cp*-free cobalt-catalyzed C−H bond functionalization, see:
- 8aL. Grigorjeva, O. Daugulis, Angew. Chem. Int. Ed. 2014, 53, 10209–10212; Angew. Chem. 2014, 126, 10373–10376;
- 8bW. Ma, L. Ackermann, ACS Catal. 2015, 5, 2822–2825;
- 8cX. Wu, K. Yang, Y. Zhao, H. Sun, G. Li, H. Ge, Nat. Commun. 2015, 6, 6462;
- 8dO. Planas, C. J. Whiteoak, A. Company, X. Ribas, Adv. Synth. Catal. 2015, 357, 4003–4012;
- 8eJ. Zhang, H. Chen, C. Lin, Z. Liu, C. Wang, Y. Zhang, J. Am. Chem. Soc. 2015, 137, 12990–12996;
- 8fD. Kalsi, B. Sundararaju, Org. Lett. 2015, 17, 6118–6121;
- 8gL.-B. Zhang, X.-Q. Hao, Z.-J. Liu, X.-X. Zheng, S.-K. Zhang, J.-L. Niu, M.-P. Song, Angew. Chem. Int. Ed. 2015, 54, 10012–10015; Angew. Chem. 2015, 127, 10150–10153;
- 8hR. Mei, H. Wang, S. Warratz, S. A. Macgregor, L. Ackermann, Chem. Eur. J. 2016, 22, 6759–6763;
- 8iV. G. Landge, G. Jaiswal, E. Balaraman, Org. Lett. 2016, 18, 812–815;
- 8jP. Gandeepan, P. Rajamalli, C.-H. Cheng, Angew. Chem. Int. Ed. 2016, 55, 4308–4311; Angew. Chem. 2016, 128, 4380–4383;
- 8kS. Maity, R. Kancherla, U. Dhawa, E. Hoque, S. Pimparkar, D. Maiti, ACS Catal. 2016, 6, 5493–5499.
- 9
- 9aL.-B. Zhang, X.-Q. Hao, S.-K. Zhang, Z.-J. Liu, X.-X. Zheng, J.-F. Gong, J.-L. Niu, M.-P. Song, Angew. Chem. Int. Ed. 2015, 54, 272–275; Angew. Chem. 2015, 127, 274–277;
- 9bL. B. Zhang, S.-K. Zhang, D. Wei, X. Zhu, X.-Q. Hao, J.-H. Su, J.-L. Niu, M.-P. Song, Org. Lett. 2016, 18, 1318–1321.
- 10X.-K. Guo, L.-B. Zhang, D. Wei, J.-L. Niu, Chem. Sci. 2015, 6, 7059–7071.
- 11For selected examples of [Cp*CoIII]-catalyzed C−H bond functionalization, see:
- 11aT. Yoshino, H. Ikemoto, S. Matsunaga, M. Kanai, Chem. Eur. J. 2013, 19, 9142–9146;
- 11bT. Yoshino, H. Ikemoto, S. Matsunaga, M. Kanai, Angew. Chem. Int. Ed. 2013, 52, 2207–2211; Angew. Chem. 2013, 125, 2263–2267;
- 11cD.-G. Yu, T. Gensch, F. de Azambuja, S. Vásquez-Céspedes, F. Glorius, J. Am. Chem. Soc. 2014, 136, 17722–17725;
- 11dE. Ozkal, B. Cacherat, B. Morandi, ACS Catal. 2015, 5, 6458–6462;
- 11eJ. R. Hummel, J. A. Ellman, J. Am. Chem. Soc. 2015, 137, 490–498;
- 11fB. Sun, T. Yoshino, M. Kanai, S. Matsunaga, Angew. Chem. Int. Ed. 2015, 54, 12968–12972; Angew. Chem. 2015, 127, 13160–13164;
- 11gJ. Park, S. Chang, Angew. Chem. Int. Ed. 2015, 54, 14103–14107; Angew. Chem. 2015, 127, 14309–14313;
- 11hM. Sen, B. Emayavaramban, N. Barsu, J. R. Premkumar, B. Sundararaju, ACS Catal. 2016, 6, 2792–2796;
- 11iD. Zell, Q. Bu, M. Feldt, L. Ackermann, Angew. Chem. Int. Ed. 2016, 55, 7408–7412; Angew. Chem. 2016, 128, 7534–7538;
- 11jY. Liang, N. Jiao, Angew. Chem. Int. Ed. 2016, 55, 4035–4039; Angew. Chem. 2016, 128, 4103–4107;
- 11kS. Prakash, K. Muralirajan, C.-H. Cheng, Angew. Chem. Int. Ed. 2016, 55, 1844–1848; Angew. Chem. 2016, 128, 1876–1880;
- 11lR. Kuppusamy, K. Muralirajan, C.-H. Cheng, ACS Catal. 2016, 6, 3909–3913;
- 11mK. Muralirajan, R. Kuppusamy, S. Prakash, C.-H. Cheng, Adv. Synth. Catal. 2016, 358, 774–783;
- 11nT. Gensch, F. J. R. Klauck, F. Glorius, Angew. Chem. Int. Ed. 2016, 55, 11287–11291; Angew. Chem. 2016, 128, 11457–11461.
- 12CCDC 1497948 (3 aa), 1497950 (5) and 1497951 (6) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 13For copper-mediated direct C−H/C−H couplings of amides with acidic azoles assisted by 8-aminoquinoline, see:
- 13aM. Nishino, K. Hirano, T. Satoh, M. Miura, Angew. Chem. Int. Ed. 2013, 52, 4457–4461; Angew. Chem. 2013, 125, 4553–4557. During the preparation of this manuscript, the group of You reported a cobalt-catalyzed heteroarylation of amides with acidic azoles assisted by the same bidentate directing group:
- 13bG. Tan, S. He, X. Huang, X. Liao, Y. Cheng, J. You, Angew. Chem. Int. Ed. 2016, 55, 10414–10418; Angew. Chem. 2016, 128, 10570–10574.
- 14At present, other pathways could not be completely excluded, including: 1) two C−H activation steps occurring within a single cobalt center in a sequential manner. 2) CoI/CoIII cycle (see Ref. [13b]): 1 a undergoes an intramolecular SET process and an intermolecular SET process to form a cyclometalated CoIII intermediate.