Closing Uranyl Polyoxometalate Capsules with Bismuth and Lead Polyoxocations
Graphical Abstract
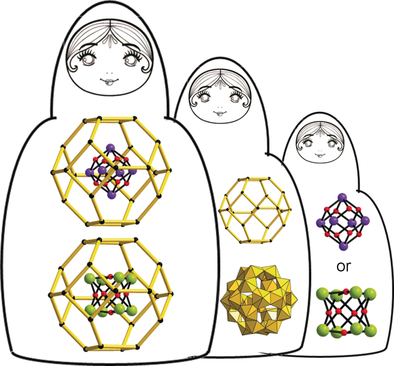
Heavy and heavier: Polyoxocations of the heavy metals bismuth and lead were nested inside uranyl polyoxoanion capsules, creating very distinctive X-ray scattering profiles. Matching symmetry and electrostatic attraction between the core and shell clusters enabled the formation of endohedral Pb@U24 and Bi@U24 in high yield and purity.
Abstract
Uranyl polyoxometalate clusters are both fundamentally fascinating and potentially relevant to nuclear energy applications. With only ten years of development, there is still much to be discovered about heterometal derivatives and aqueous speciation and behavior. Herein, we show that it is possible to encapsulate the polyoxocations [Bi6O8]2+ and [Pb8O6]4+ in [(UO2)(O2)(OH)]2424− (denoted Bi@U24 and Pb@U24) in pure form and high yields despite the fact that under aqueous conditions, these compounds are stable on opposite ends of the pH scale. Moreover, [Pb8O6]4+ is a formerly unknown PbII polynuclear species, both in solution and in the solid state. Raman spectroscopic and mass spectrometric analysis of the reaction solutions revealed the very rapid assembly of the nested clusters, driven by bismuth- or lead-promoted decomposition of excess peroxide, which inhibits U24 formation. Experimental and simulated small-angle X-ray scattering data of Bi@U24 and Pb@U24 solutions revealed that this technique is very sensitive not only to the size and shape of the clusters, but also to the encapsulated species.



 m, a=b=c=33.5330(6) Å, α=β=γ=90°, V=37706.6(12) Å3, ρcalc=2.622 g cm−3, μ(CuKα)=53.461 cm−1, 120 824 reflections measured on an X8 Bruker CCD diffractometer at 173(2) K, R1=0.0821, wR2=0.1965, GOF=1.209 for 3218 independent reflections with I>2σ(I). Depository number CSD-431617.
m, a=b=c=33.5330(6) Å, α=β=γ=90°, V=37706.6(12) Å3, ρcalc=2.622 g cm−3, μ(CuKα)=53.461 cm−1, 120 824 reflections measured on an X8 Bruker CCD diffractometer at 173(2) K, R1=0.0821, wR2=0.1965, GOF=1.209 for 3218 independent reflections with I>2σ(I). Depository number CSD-431617.

