The Organocatalytic Approach to Enantiopure 2H- and 3H-Pyrroles: Inhibitors of the Hedgehog Signaling Pathway
Lisa Kötzner
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorDr. Markus Leutzsch
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorDr. Sonja Sievers
Max-Planck-Institut für Molekulare Physiologie, Compound Management and Screening Center (COMAS), Otto-Hahn-Strasse 11, 44227 Dortmund, Germany
Search for more papers by this authorSumersing Patil
Max-Planck-Institut für Molekulare Physiologie, Otto-Hahn-Strasse 11, 44227 Dortmund, Germany
Search for more papers by this authorProf. Dr. Herbert Waldmann
Max-Planck-Institut für Molekulare Physiologie, Otto-Hahn-Strasse 11, 44227 Dortmund, Germany
Search for more papers by this authorDr. Yiying Zheng
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorProf. Dr. Walter Thiel
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Benjamin List
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorLisa Kötzner
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorDr. Markus Leutzsch
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorDr. Sonja Sievers
Max-Planck-Institut für Molekulare Physiologie, Compound Management and Screening Center (COMAS), Otto-Hahn-Strasse 11, 44227 Dortmund, Germany
Search for more papers by this authorSumersing Patil
Max-Planck-Institut für Molekulare Physiologie, Otto-Hahn-Strasse 11, 44227 Dortmund, Germany
Search for more papers by this authorProf. Dr. Herbert Waldmann
Max-Planck-Institut für Molekulare Physiologie, Otto-Hahn-Strasse 11, 44227 Dortmund, Germany
Search for more papers by this authorDr. Yiying Zheng
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorProf. Dr. Walter Thiel
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Benjamin List
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany
Search for more papers by this authorGraphical Abstract
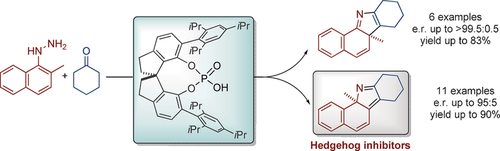
The versatility of pyrroles: A Fischer indolization and a [1,5]-alkyl shift were used in the title reaction to obtain enantioenriched 2H- or 3H-pyrroles. The products could be generated in good to excellent yields and enantiomeric ratios by using a chiral SPINOL-derived phosphoric acid derivative as catalyst. Biological evaluations revealed the novel 2H-pyrroles to be potent inhibitors of the Hedgehog signaling pathway.
Abstract
A divergent approach to enantioenriched 2H- and 3H-pyrroles catalyzed by a spirocyclic phosphoric acid is reported that makes use of a Fischer-type indolization and a [1,5]-alkyl shift. Catalyzed by the chiral phosphoric acid STRIP, good to excellent yields and enantioselectivities could be obtained. Remarkably, biological evaluation reveals one of these novel 2H-pyrroles to be a potent but nontoxic inhibitor of the Hedgehog signaling pathway by binding to the Smoothened protein.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201602932-sup-0001-misc_information.pdf3.8 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. Yuan, S. Venkatraman, Y. Zheng, B. M. McKeever, L. W. Dillard, S. B. Singh, J. Med. Chem. 2013, 56, 4156–4180;
- 1bB.-M. Swahn, K. Kolmodin, S. Karlström, S. von Berg, P. Söderman, J. Holenz, S. Berg, J. Lindström, M. Sundström, D. Turek, J. Kihlström, C. Slivo, L. Andersson, D. Pyring, D. Rotticci, L. Öhberg, A. Kers, K. Bogar, F. von Kieseritzky, M. Bergh, L.-L. Olsson, J. Janson, S. Eketjäll, B. Georgievska, F. Jeppsson, J. Fälting, J. Med. Chem. 2012, 55, 9346–9361;
- 1cA. Lindgren, G. Eklund, D. Turek, J. Malmquist, B.-M. Swahn, J. Holenz, S. von Berg, S. Karlström, T. Bueters, Drug Metab. Dispos. 2013, 41, 1134–1147;
- 1dG. Cirrincione, A. M. Almerico, G. Dattolo, E. Aiello, S. Grimaudo, P. Diana, F. Misuraca, Farmaco 1992, 47, 1555–1562;
- 1eG. Cirrincione, A. M. Almerico, S. Grimaudo, P. Diana, F. Mingoia, P. Barraja, F. Misuraca, Farmaco 1996, 51, 49–52;
- 1fV. Padmavathi, T. Radha Lakshmi, K. Mahesh, A. Padmaja, Chem. Pharm. Bull. 2009, 57, 1200–1205;
- 1gJ. T. Gupton, Topics in Heterocyclic Chemistry, Vol. 2, Springer, Berlin, 2006, pp. 53–92;
- 1hY.-M. Zhang, N.-H. Tan, Y. Lu, Y. Chang, R.-R. Jia, Org. Lett. 2007, 9, 4579–4581;
- 1iS. Saito, T. Kubota, J. i. Kobayashi, Tetrahedron Lett. 2007, 48, 5693–5695;
- 1jE. Deery, S. Schroeder, A. D. Lawrence, S. L. Taylor, A. Seyedarabi, J. Waterman, K. S. Wilson, D. Brown, M. A. Geeves, M. J. Howard, R. W. Pickersgill, M. J. Warren, Nat. Chem. Biol. 2012, 8, 933–940;
- 1kD. Thibaut, L. Debussche, F. Blanche, Proc. Natl. Acad. Sci. USA 1990, 87, 8795–8799.
- 2
- 2aC.-X. Zhuo, W.-B. Liu, Q.-F. Wu, S.-L. You, Chem. Sci. 2012, 3, 205–208;
- 2bC.-X. Zhuo, Y. Zhou, S.-L. You, J. Am. Chem. Soc. 2014, 136, 6590–6593;
- 2cJ.-Y. Liao, P.-L. Shao, Y. Zhao, J. Am. Chem. Soc. 2015, 137, 628–631;
- 2dC.-X. Zhuo, Q. Cheng, W.-B. Liu, Q. Zhao, S.-L. You, Angew. Chem. Int. Ed. 2015, 54, 8475–8479; Angew. Chem. 2015, 127, 8595–8599.
- 3S. Huang, L. Kötzner, C. K. De, B. List, J. Am. Chem. Soc. 2015, 137, 3446–3449.
- 4
- 4aS. Müller, M. J. Webber, B. List, J. Am. Chem. Soc. 2011, 133, 18534–18537;
- 4bA. Martínez, M. J. Webber, S. Müller, B. List, Angew. Chem. Int. Ed. 2013, 52, 9486–9490; Angew. Chem. 2013, 125, 9664–9668;
- 4cL. Kötzner, M. J. Webber, A. Martínez, C. De Fusco, B. List, Angew. Chem. Int. Ed. 2014, 53, 5202–5205; Angew. Chem. 2014, 126, 5303–5306;
- 4dC. K. De, F. Pesciaioli, B. List, Angew. Chem. Int. Ed. 2013, 52, 9293–9295; Angew. Chem. 2013, 125, 9463–9465;
- 4eG.-Q. Li, H. Gao, C. Keene, M. Devonas, D. H. Ess, L. Kürti, J. Am. Chem. Soc. 2013, 135, 7414–7417;
- 4fA. W. Schammel, B. W. Boal, L. Zu, T. Mesganaw, N. K. Garg, Tetrahedron 2010, 66, 4687–4695.
- 5For an enantioselective synthesis of related indolenines by the Pd-catalyzed dearomatization of naphthalenes, see J. García-Fortanet, F. Kessler, S. L. Buchwald, J. Am. Chem. Soc. 2009, 131, 6676–6677.
- 6
- 6aP.-K. Chiu, K.-H. Lui, P. N. Maini, M. P. Sammes, J. Chem. Soc. Chem. Commun. 1987, 109–110;
- 6bK.-H. Lui, M. P. Sammes, J. Chem. Soc. Perkin Trans. 1 1990, 457–468;
- 6cA. A. Shimkin, V. Z. Shirinian, D. M. Nikalin, M. M. Krayushkin, T. S. Pivina, N. A. Troitsky, L. G. Vorontsova, Z. A. Starikova, Eur. J. Org. Chem. 2006, 2087–2092;
- 6dJ. L. Wong, M. H. Ritchie, C. M. Gladstone, J. Chem. Soc. D 1971, 1093–1094.
10.1039/C2971001093B Google Scholar
- 7For recent studies by the pioneers of asymmetric phosphoric acid catalysis, see
- 7aT. Akiyama, Synlett 2016, 542–545;
- 7bK. Kanomata, M. Terada, Synlett 2016, 581–585.
- 8The amount of 2H-pyrrole 5 has an influence on the enantioselectivity of 3H-pyrrole 4 as a result of a kinetic resolution as background reaction. Under our optimized conditions, 2H-pyrroles 5 were obtained in only trace amounts and their formation was found to be temperature-dependent, whereas the amount of benzoic acid had no influence on their generation (see the Supporting Information).
- 9SPINOL-derived phosphoric acids were independently introduced by three research groups:
- 9aF. Xu, D. Huang, C. Han, W. Shen, X. Lin, Y. Wang, J. Org. Chem. 2010, 75, 8677–8680;
- 9bI. Čorić, S. Müller, B. List, J. Am. Chem. Soc. 2010, 132, 17370–17373;
- 9cC.-H. Xing, Y.-X. Liao, J. Ng, Q.-S. Hu, J. Org. Chem. 2011, 76, 4125–4131.
- 10Cooperative effects between carboxylic acids and phosphoric acids in organocatalysis have been described:
- 10aM. R. Monaco, B. Poladura, M. Diaz de Los Bernardos, M. Leutzsch, R. Goddard, B. List, Angew. Chem. Int. Ed. 2014, 53, 7063–7067; Angew. Chem. 2014, 126, 7183–7187;
- 10bG. Li, J. C. Antilla, Org. Lett. 2009, 11, 1075–1078;
- 10cM. Rueping, C. Azap, Angew. Chem. Int. Ed. 2006, 45, 7832–7835; Angew. Chem. 2006, 118, 7996–7999;
- 10dT. Akiyama, Y. Tamura, J. Itoh, H. Morita, K. Fuchibe, Synlett 2006, 0141–0143;
- 10eM. Rueping, E. Sugiono, F. R. Schoepke, Synlett 2007, 1441–1445; for an account, see
- 10fM. R. Monaco, G. Pupo, B. List, Synlett 2016, 1027–1040.
- 11
- 11aThe use of heterocyclic ketones led to reactivity and selectivity issues;
- 11bthe use of acyclic ketones was not possible because of reactivity issues.
- 12
- 12aF. A. Carey, R. J. Sundberg, Advanced Organic Chemistry: Part A: Structure and Mechanisms, Springer, New York, 2007;
- 12bB. A. Hess, L. J. Schaad, J. Pancir, J. Am. Chem. Soc. 1985, 107, 149–154;
- 12cR. B. Woodward, R. Hoffmann, J. Am. Chem. Soc. 1965, 87, 2511–2513;
- 12dR. B. Woodward, R. Hoffmann, Angew. Chem. Int. Ed. Engl. 1969, 8, 781–853; Angew. Chem. 1969, 81, 797–869;
- 12eW. H. N. Nijhuis, W. Verboom, D. N. Reinhoudt, S. Harkema, J. Am. Chem. Soc. 1987, 109, 3136–3138;
- 12fW. H. N. Nijhuis, W. Verboom, A. Abu El-Fadl, G. J. Van Hummel, D. N. Reinhoudt, J. Org. Chem. 1989, 54, 209–216;
- 12gJ. Mulzer, U. Kühl, G. Huttner, K. Evertz, Chem. Ber. 1988, 121, 2231–2238;
- 12hW. R. Roth, J. König, K. Stein, Chem. Ber. 1970, 103, 426–439.
- 13H. B. Kagan, J. C. Fiaud, Topics in Stereochemistry, Wiley, Hoboken, 1988, pp. 249–330.
10.1002/9780470147276.ch4 Google Scholar
- 14
- 14aG. Bringmann, T. Bruhn, K. Maksimenka, Y. Hemberger, Eur. J. Org. Chem. 2009, 2009, 2717–2727;
- 14bT. Bruhn, A. Schaumlöffel, Y. Hemberger, G. Bringmann, Chirality 2013, 25, 243–249.
- 15For reviews, see
- 15aL. L. Rubin, F. J. de Sauvage, Nat. Rev. Drug Discovery 2006, 5, 1026–1033;
- 15bJ. M. Y. Ng, T. Curran, Nat. Rev. Cancer 2011, 11, 493–501;
- 15cS. Peukert, K. Miller-Moslin, ChemMedChem 2010, 5, 500–512;
- 15dM. R. Tremblay, K. McGovern, M. A. Read, A. C. Castro, Curr. Opin. Chem. Biol. 2010, 14, 428–435;
- 15eB. Z. Stanton, L. F. Peng, Mol. BioSyst. 2010, 6, 44–54;
- 15fN. Mahindroo, C. Punchihewa, N. Fujii, J. Med. Chem. 2009, 52, 3829–3845;
- 15gP. Heretsch, L. Tzagkaroulaki, A. Giannis, Bioorg. Med. Chem. 2010, 18, 6613–6624;
- 15hC. Nusslein-Volhard, E. Wieschaus, Nature 1980, 287, 795–801.
- 16For selected examples, see
- 16aH. Takayama, Z.-J. Jia, L. Kremer, J. O. Bauer, C. Strohmann, S. Ziegler, A. P. Antonchick, H. Waldmann, Angew. Chem. Int. Ed. 2013, 52, 12404–12408; Angew. Chem. 2013, 125, 12630–12634;
- 16bS. Sinha, J. K. Chen, Nat. Chem. Biol. 2006, 2, 29–30;
- 16cJ. K. Chen, J. Taipale, M. K. Cooper, P. A. Beachy, Genes Dev. 2002, 16, 2743–2748;
- 16dA. Büttner, K. Seifert, T. Cottin, V. Sarli, L. Tzagkaroulaki, S. Scholz, A. Giannis, Bioorg. Med. Chem. 2009, 17, 4943–4954;
- 16eK. Seifert, A. Büttner, S. Rigol, N. Eilert, E. Wandel, A. Giannis, Bioorg. Med. Chem. 2012, 20, 6465–6481;
- 16fJ. K. Chen, J. Taipale, K. E. Young, T. Maiti, P. A. Beachy, Proc. Natl. Acad. Sci. USA 2002, 99, 14071–14076.
- 17
- 17aT. Nakamura, T. Aikawa, M. Iwamoto-Enomoto, M. Iwamoto, Y. Higuchi, P. Maurizio, N. Kinto, A. Yamaguchi, S. Noji, K. Kurisu, T. Matsuya, Biochem. Biophys. Res. Commun. 1997, 237, 465–469;
- 17bX. Wu, J. Walker, J. Zhang, S. Ding, P. G. Schultz, Chem. Biol. 2004, 11, 1229–1238.
- 18Biological evaluations showed no significant difference between rac-, (R)-, and (S)-5 b (see the Supporting Information).