Structural Revisions of a Class of Natural Products: Scaffolds of Aglycon Analogues of Fusicoccins and Cotylenins Isolated from Fungi
Correction(s) for this article
-
Corrigendum: Structural Revisions of a Class of Natural Products: Scaffolds of Aglycon Analogues of Fusicoccins and Cotylenins Isolated from Fungi
- Ying Tang,
- Yongbo Xue,
- Guang Du,
- Jianping Wang,
- Junjun Liu,
- Bin Sun,
- Xiao-Nian Li,
- Guangmin Yao,
- Zengwei Luo,
- Yonghui Zhang,
- Volume 57Issue 46Angewandte Chemie International Edition
- pages: 14970-14970
- First Published online: September 11, 2018
Dr. Ying Tang
Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
These authors contributed equally to this work.
Search for more papers by this authorDr. Yongbo Xue
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030 China
These authors contributed equally to this work.
Search for more papers by this authorProf. Guang Du
Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
These authors contributed equally to this work.
Search for more papers by this authorProf. Jianping Wang
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030 China
Search for more papers by this authorProf. Junjun Liu
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030 China
Search for more papers by this authorBin Sun
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030 China
Search for more papers by this authorDr. Xiao-Nian Li
State Key Laboratory of Phytochemistry and Plant Resources in West China, Chinese Academy of Sciences, Kunming, China
Search for more papers by this authorProf. Guangmin Yao
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030 China
Search for more papers by this authorDr. Zengwei Luo
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030 China
Search for more papers by this authorCorresponding Author
Prof. Yonghui Zhang
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030 China
Search for more papers by this authorDr. Ying Tang
Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
These authors contributed equally to this work.
Search for more papers by this authorDr. Yongbo Xue
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030 China
These authors contributed equally to this work.
Search for more papers by this authorProf. Guang Du
Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
These authors contributed equally to this work.
Search for more papers by this authorProf. Jianping Wang
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030 China
Search for more papers by this authorProf. Junjun Liu
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030 China
Search for more papers by this authorBin Sun
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030 China
Search for more papers by this authorDr. Xiao-Nian Li
State Key Laboratory of Phytochemistry and Plant Resources in West China, Chinese Academy of Sciences, Kunming, China
Search for more papers by this authorProf. Guangmin Yao
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030 China
Search for more papers by this authorDr. Zengwei Luo
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030 China
Search for more papers by this authorCorresponding Author
Prof. Yonghui Zhang
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030 China
Search for more papers by this authorGraphical Abstract
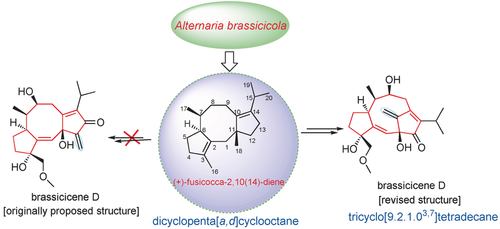
Brass tacks: A class of natural products (NPs) regarding the brassicicene C-type diterpenoids was revised and characterized as the first class of bridgehead double-bond-containing NPs with a bicyclo[6.2.1]undecane carbon skeleton. This study shows the potential of the application of computational prediction methods and biosynthetic logic-based structure elucidation to determining the structure and stability of NPs.
Abstract
The reisolation and structural revision of brassicicene D is described, and inspired us to reassign the core skeletons of brassicicenes C–H, J and K, ranging from dicyclopenta[a,d]cyclooctane to tricyclo[9.2.1.03,7]tetradecane using quantum-chemical predictions and experimental validation strategies. Three novel, highly modified fusicoccanes, brassicicenes L–N, were also isolated from the fungus Alternaria brassicicola, and their structures were unequivocally established by spectroscopic data, ECD calculations, and crystallography. The reassigned structures represent the first class of bridgehead double-bond-containing natural products with a bicyclo[6.2.1]undecane carbon skeleton. Furthermore, their stabilities were first predicted with olefin strain energy calculations. Collectively, these findings extend our view of the application of computational predictions and biosynthetic logic-based structure elucidation to address problems related to the structure and stability of natural products.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201600313-sup-0001-misc_information.pdf6.7 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1F. E. Koehn, G. T. Carter, Nat. Rev. Drug Discovery 2005, 4, 206–220.
- 2
- 2aJ. Clardy, C. Walsh, Nature 2004, 432, 829–837;
- 2bB. L. Prosser, C. W. Ward, W. J. Lederer, Science 2011, 333, 1440–1445;
- 2cA. H. De Boer, I. J. de Vries-van Leeuwen, Trends Plant Sci. 2012, 17, 360–368.
- 3
- 3aK. D. Barrow, D. H. R. Barton, E. B. Chain, U. F. W. Ohnsorge, R. Thomas, Chem. Commun. 1968, 1198–1200;
- 3bE. Hough, M. B. Hursthouse, S. Neidle, D. Rogers, Chem. Commun. 1968, 1197–1198;
- 3cA. Ballio, M. Brufani, C. G. Casinovi, S. Cerrini, W. Fedeli, R. Pellicciari, B. Santurbano, A. Vaciago, Experientia 1968, 24, 631–635.
- 4D. R. Williams, L. A. Robinson, C. R. Nevill, J. P. Reddy, Angew. Chem. Int. Ed. 2007, 46, 915–918; Angew. Chem. 2007, 119, 933–936.
- 5B. De Boer, Trends Plant Sci. 1997, 2, 60–66.
- 6
- 6aT. Toyomasu, M. Tsukahara, A. Kaneko, R. Niida, W. Mitsuhashi, T. Dairi, N. Kato, T. Sassa, Proc. Natl. Acad. Sci. USA 2007, 104, 3084–3088;
- 6bR. Rose, S. Erdmann, S. Bovens, A. Wolf, M. Rose, S. Hennig, H. Waldmann, C. Ottmann, Angew. Chem. Int. Ed. 2010, 49, 4129–4132; Angew. Chem. 2010, 122, 4223–4226;
- 6cM. Skwarczynska, M. Molzan, C. Ottmann, Proc. Natl. Acad. Sci. USA 2013, 110, 377–386;
- 6dP. Parvatkar, N. Kato, M. Uesugi, S. Sato, J. Ohkanda, J. Am. Chem. Soc. 2015, 137, 15624–15627.
- 7M. B. Yaffe, K. Rittinger, S. Volinia, P. R. Caron, A. Aitken, H. Leffers, S. J. Gamblin, S. J. Smerdon, L. C. Cantley, Cell 1997, 91, 961–971.
- 8K. Yamada, Y. Honma, K. i. Asahi, T. Sassa, K. i. Hino, S. Tomoyasu, Br. J. Haematol. 2001, 114, 814–821.
- 9
- 9aT. Kasukabe, J. Okabe-Kado, Y. Honma, Cancer Sci. 2008, 99, 1693–1698;
- 9bI. J. De Vries-van Leeuwen, C. Kortekaas-Thijssen, J. A. N. Mandouckou, S. Kas, A. Evidente, A. H. De Boer, Cancer Lett. 2010, 293, 198–206.
- 10T. D. Bunney, A. H. De Boer, M. Levin, Development 2003, 130, 4847–4858.
- 11L. Camoni, C. Di Lucente, S. Visconti, P. Aducci, Biochem. J. 2011, 436, 429–436.
- 12Our SciFinder search terms included: fusicoccane, cotylenin, brassicicene, fusicoplagin, dicyclopenta[a,d]cyclooctane, 5-8-5 carbocyclic, ophiobolin, and ceroplastols. Interestingly, despite quite a different set of results produced by each of these searches, and in all more than 300 diterpenoids and diterpenoid glycosides and 30 fusicoccane sesterterpenes were obtained for the period of 1962 to 2015.
- 13T. Miyake, Y. Honma, T. Urano, N. Kato, J. Suzumiya, Int. J. Oncol. 2015, 47, 315–324.
- 14G. Subbarao, K. Nakahara, M. d. P. Hurtado, H. Ono, D. Moreta, A. F. Salcedo, A. Yoshihashi, T. Ishikawa, M. Ishitani, M. Ohnishi-Kameyama, Proc. Natl. Acad. Sci. USA 2009, 106, 17302–17307.
- 15
- 15aA. K. M. Takahashi, N. Kato, T. Nishi, I. Hamachi, J. Ohkanda, Angew. Chem. Int. Ed. 2012, 51, 509–512; Angew. Chem. 2012, 124, 524–527;
- 15bL. G. Milroy, L. Brunsveld, C. Ottmann, ACS Chem. Biol. 2012, 8, 27–35.
- 16
- 16aY. Ono, A. Minami, M. Noike, Y. Higuchi, T. Toyomasu, T. Sassa, N. Kato, T. Dairi, J. Am. Chem. Soc. 2011, 133, 2548–2555;
- 16bS. L. MacKinnon, P. Keifer, W. A. Ayer, Phytochemistry 1999, 51, 215–221;
- 16cM. S. C. Pedras, P. B. Chumala, W. Jin, M. S. Islam, D. W. Hauck, Phytochemistry 2009, 70, 394–402;
- 16dS. T. H. Kenmoku, M. Oogushi, Y. Yagi, T. Sassa, M. Toyota, Y. Asakawa, Nat. Prod. Commun. 2014, 9, 351–354;
- 16eA. Minami, N. Tajima, Y. Higuchi, T. Toyomasu, T. Sassa, N. Kato, T. Dairi, Bioorg. Med. Chem. Lett. 2009, 19, 870–874;
- 16fM. Hashimoto, Y. Higuchi, S. Takahashi, H. Osada, T. Sakaki, T. Toyomasu, T. Sassa, N. Kato, T. Dairi, Bioorg. Med. Chem. Lett. 2009, 19, 5640–5643.
- 17K. Nicolaou, S. A. Snyder, Angew. Chem. Int. Ed. 2005, 44, 1012–1044; Angew. Chem. 2005, 117, 1036–1069.
- 18
- 18aR. C. Breton, W. F. Reynolds, Nat. Prod. Rep. 2013, 30, 501–524;
- 18bJ. Yang, S. X. Huang, Q. S. Zhao, J. Phys. Chem. A 2008, 112, 12132–12139.
- 19DFT Calculations were performed with Gaussian 09, Wallingford CT, 2010. See the Supporting Information for ECD calculations and full crystallographic details.
- 20D. R. Williams, R. W. Heidebrecht, J. Am. Chem. Soc. 2003, 125, 1843–1850.
- 21J. Y. Mak, R. H. Pouwer, C. M. Williams, Angew. Chem. Int. Ed. 2014, 53, 13664–13688; Angew. Chem. 2014, 126, 13882–13906.
- 22
- 22aD. J. Martella, M. Jones Jr, P. V. R. Schleyer, W. F. Maier, J. Am. Chem. Soc. 1979, 101, 7634–7637;
- 22bW. F. Maier, P. v. R. Schleyer, J. Am. Chem. Soc. 1981, 103, 1891–1900.
- 23E. H. Krenske, C. M. Williams, Angew. Chem. Int. Ed. 2015, 54, 10608–10612; Angew. Chem. 2015, 127, 10754–10758.
- 24A. Monks, D. Scudiero, P. Skehan, R. Shoemaker, K. Paull, D. Vistica, C. Hose, J. Langley, P. Cronise, A. Vaigro-Wolff, J. Natl. Cancer Inst. 1991, 83, 757–766.
- 25T. P. Stockdale, C. M. Williams, Chem. Soc. Rev. 2015, 44, 7737–7763.
- 26L. A. Paquette, Chem. Soc. Rev. 1995, 24, 9–17.
- 27A. Hoffmann-Röder, N. Krause, Angew. Chem. Int. Ed. 2004, 43, 1196–1216; Angew. Chem. 2004, 116, 1216–1236.