α-Arylation of Ketimines with Aryl Sulfides at a Low Palladium Catalyst Loading
Dr. Ke Gao
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Hideki Yorimitsu
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorProf. Dr. Atsuhiro Osuka
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorDr. Ke Gao
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Hideki Yorimitsu
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorProf. Dr. Atsuhiro Osuka
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorGraphical Abstract
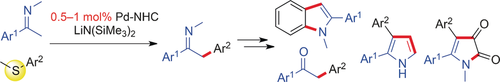
Aryl sulfides, although typically poisonous to transition-metal catalysts, serve as aryl electrophiles in the catalytic α-arylation of ketimines. Low catalyst loadings (down to 0.5 mol %) of a Pd–NHC complex are sufficient for efficient arylation. α-Arylated ketimine products were used to synthesize various azaarenes including 2,3-diarylpyrroles.
Abstract
Instead of using aryl halides, aryl sulfides, typically poisonous to transition-metal catalysts, were found to serve as aryl electrophiles in the catalytic α-arylation of ketimines, a class of carbonyl derivatives. Low catalyst loadings (down to 0.5 mol %) of a palladium–NHC complex are sufficient for efficient arylation. α-Arylated ketimine products are useful for the synthesis of various azaarenes, including 2,3-diarylpyrroles, an indole, and pyrrolediones.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201600248-sup-0001-misc_information.pdf6.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see:
- 1aS. T. Sivanandan, A. Shaji, I. Ibnusaud, C. C. C. J. Seechurn, T. J. Colacot, Eur. J. Org. Chem. 2015, 2015, 38;
- 1bH. K. Potukuchi, A. P. Spork, T. J. Donohoe, Org. Biomol. Chem. 2015, 13, 4367;
- 1cP. Novak, R. Martin, Curr. Org. Chem. 2011, 15, 3233;
- 1dF. Bellina, R. Rossi, Chem. Rev. 2010, 110, 1082;
- 1eC. C. C. Johansson, T. J. Colacot, Angew. Chem. Int. Ed. 2010, 49, 676; Angew. Chem. 2010, 122, 686;
- 1fD. A. Culkin, J. F. Hartwig, Acc. Chem. Res. 2003, 36, 234.
- 2
- 2aM. Palucki, S. L. Buchwald, J. Am. Chem. Soc. 1997, 119, 11108;
- 2bB. C. Hamann, J. F. Hartwig, J. Am. Chem. Soc. 1997, 119, 12382;
- 2cT. Satoh, Y. Kawamura, M. Miura, M. Nomura, Angew. Chem. Int. Ed. Engl. 1997, 36, 1740; Angew. Chem. 1997, 109, 1820.
- 3For selected recent examples, see:
- 3aM. P. Drapeau, I. Fabre, L. Grimaud, I. Ciofini, T. Ollevier, M. Taillefer, Angew. Chem. Int. Ed. 2015, 54, 10587; Angew. Chem. 2015, 127, 10733;
- 3bJ. A. Fernández-Salas, E. Marelli, D. B. Cordes, A. M. Z. Slawin, S. P. Nolan, Chem. Eur. J. 2015, 21, 3906;
- 3cB. Zheng, T. Jia, P. J. Walsh, Adv. Synth. Catal. 2014, 356, 165;
- 3dP. G. Alsabeh, M. Stradiotto, Angew. Chem. Int. Ed. 2013, 52, 7242; Angew. Chem. 2013, 125, 7383;
- 3eT. Hama, S. Ge, J. F. Hartwig, J. Org. Chem. 2013, 78, 8250;
- 3fB. Zhang, T. Jia, P. J. Walsh, Org. Lett. 2013, 15, 4190;
- 3gL. Ackermann, V. P. Mehta, Chem. Eur. J. 2012, 18, 10230;
- 3hK. D. Hesp, R. J. Lundgren, M. Stradiotto, J. Am. Chem. Soc. 2011, 133, 5194.
- 4R. Takise, K. Muto, J. Yamaguchi, K. Itami, Angew. Chem. Int. Ed. 2014, 53, 6791; Angew. Chem. 2014, 126, 6909.
- 5
- 5aI. P. Beletskaya, V. P. Ananikov, Eur. J. Org. Chem. 2007, 3431;
- 5bT. Kondo, T. Mitsudo, Chem. Rev. 2000, 100, 3205;
- 5cI. P. Beletskaya, V. P. Ananikov, Chem. Rev. 2011, 111, 1596;
- 5dC. C. Eichman, J. P. Stambuli, Molecules 2011, 16, 590;
- 5eH. Liu, X. Feng, Chem. Asian J. 2013, 8, 2546;
- 5fC.-F. Lee, Y.-C. Liu, S. S. Badsara, Chem. Asian J. 2014, 9, 706.
- 6For selected reviews, see:
- 6aS. R. Dubbaka, P. Vogel, Angew. Chem. Int. Ed. 2005, 44, 7674; Angew. Chem. 2005, 117, 7848;
- 6bH. Prokopcová, C. O. Kappe, Angew. Chem. Int. Ed. 2008, 47, 3674; Angew. Chem. 2008, 120, 3732;
- 6cH. Prokopcová, C. O. Kappe, Angew. Chem. Int. Ed. 2009, 48, 2276; Angew. Chem. 2009, 121, 2312;
- 6dE. C. Garnier-Amblard, L. S. Liebeskind, Boronic Acids, 2nd ed. ), Wiely-VCH, Weinheim, 2011, Chap. 7;
10.1002/9783527639328.ch7 Google Scholar
- 6eS. G. Modha, V. P. Mehta, E. V. Van der Eycken, Chem. Soc. Rev. 2013, 42, 5042;
- 6fL. Wang, W. He, Z. Yu, Chem. Soc. Rev. 2013, 42, 599;
- 6gF. Pan, Z.-J. Shi, ACS Catal. 2014, 4, 280. For some very recent examples, see:
- 6hA. J. Eberhart, J. E. Imbriglio, D. J. Procter, Org. Lett. 2011, 13, 5882;
- 6iL. Melzig, A. Metzger, P. Knochel, Chem. Eur. J. 2011, 17, 2948;
- 6jS. G. Modha, J. C. Trivedi, V. P. Mehta, D. S. Ermolat′ev, E. V. Van der Eycken, J. Org. Chem. 2011, 76, 846;
- 6kM. Koley, L. Wimmer, M. Schnürch, M. D. Mihovilovic, Eur. J. Org. Chem. 2011, 1972;
- 6lS. Dahbi, P. Bisseret, Eur. J. Org. Chem. 2012, 3759;
- 6mJ. F. Hooper, R. D. Young, I. Pernik, A. S. Weller, M. C. Willis, Chem. Sci. 2013, 4, 1568;
- 6nF. Pan, H. Wang, P.-X. Shen, J. Zhao, Z.-J. Shi, Chem. Sci. 2013, 4, 1573;
- 6oG. S. Creech, O. Kwon, Chem. Sci. 2013, 4, 2670;
- 6pJ.-X. Liu, Y.-J. Liu, W.-T. Du, Y. Dong, J. Liu, M. Wang, J. Org. Chem. 2013, 78, 7293.
- 7
- 7aY. Ookubo, A. Wakamiya, H. Yorimitsu, A. Osuka, Chem. Eur. J. 2012, 18, 12690;
- 7bK. Murakami, H. Yorimitsu, A. Osuka, Angew. Chem. Int. Ed. 2014, 53, 7510; Angew. Chem. 2014, 126, 7640;
- 7cT. Sugahara, K. Murakami, H. Yorimitsu, A. Osuka, Angew. Chem. Int. Ed. 2014, 53, 9329; Angew. Chem. 2014, 126, 9483;
- 7dS. Otsuka, D. Fujino, K. Murakami, H. Yorimitsu, A. Osuka, Chem. Eur. J. 2014, 20, 13146;
- 7eA. Baralle, S. Otsuka, V. Guérin, K. Murakami, H. Yorimitsu, A. Osuka, Synlett 2015, 26, 327;
- 7fK. Gao, H. Yorimitsu, A. Osuka, Eur. J. Org. Chem. 2015, 2678.
- 8Thioesters are known to be reactive cross-coupling partners that allow low catalyst loadings. See, for instance:
- 8aK. Kunchithapatham, C. C. Eichman, J. P. Stambuli, Chem. Commun. 2011, 47, 12679. In boiling toluene in 5 days, nickel-catalyzed cross-coupling desulfurization of dibenzothiophenes with Grignard reagents went to completion with 0.1–1 mol % of Ni:
- 8bJ. Torres-Nieto, A. Arévalo, P. García-Gutiérrez, A. Acosta-Ramírez, J. J. García, Organometallics 2004, 23, 4534;
- 8cJ. Torres-Nieto, A. Arévalo, J. J. García, Organometallics 2007, 26, 2228.
- 9
- 9aE. A. B. Kantchev, J. Y. Ying, Organometallics 2009, 28, 289;
- 9bG.-R. Peh, E. A. B. Kantchev, J.-C. Er, J. Y. Ying, Chem. Eur. J. 2010, 16, 4010;
- 9cC. Valente, S. Çalimsiz, K. H. Hoi, D. Mallik, M. Sayah, M. G. Organ, Angew. Chem. Int. Ed. 2012, 51, 3314; Angew. Chem. 2012, 124, 3370;
- 9dC. Valente, M. Pompeo, M. Sayah, M. G. Organ, Org. Process Res. Dev. 2014, 18, 180;
- 9eE. A. B. Kantchev, C. J. O'Brien, M. G. Organ, Aldrichimica Acta 2006, 39, 117;
- 9fM. S. Viciu, R. F. Germaneau, O. Navarro-Fernandez, E. D. Stevens, S. P. Nolan, Organometallics 2002, 21, 5470;
- 9gO. Navarro, H. Kaur, P. Mahjoor, S. P. Nolan, J. Org. Chem. 2004, 69, 3173.
- 10
- 10aJ. K. Whitesell, M. A. Whitesell, Synthesis 1983, 517;
- 10bD. Enders, L. Wortmann, R. Peters, Acc. Chem. Res. 2000, 33, 157;
- 10cS. Mangelinckx, N. Giubellina, N. D. Kimpe, Chem. Rev. 2004, 104, 2353.
- 11J. Barluenga, A. Jiménez-Aquino, C. Valdés, F. Aznar, Angew. Chem. Int. Ed. 2007, 46, 1529; Angew. Chem. 2007, 119, 1551.
- 12Each ketimine was an E isomer purely or with a contamination of a small amount of its Z isomer. For details, see the Supporting Information.
- 13Limited methods for synthesizing N-unsubstituted 2,3-diarylpyrroles are known. See:
- 13aV. N. Engel, W. Steglich, Angew. Chem. Int. Ed. Engl. 1978, 17, 676; Angew. Chem. 1978, 90, 719;
- 13bB. B. Thompson, J. Montgomery, Org. Lett. 2011, 13, 3289;
- 13cM. Zhang, X. Fang, H. Neumann, M. Beller, J. Am. Chem. Soc. 2013, 135, 11384;
- 13dM. Zhang, H. Neumann, M. Beller, Angew. Chem. Int. Ed. 2013, 52, 597; Angew. Chem. 2013, 125, 625;
- 13eS. Yu, M. Xiong, X. Xie, Y. Liu, Angew. Chem. Int. Ed. 2014, 53, 11596; Angew. Chem. 2014, 126, 11780.
- 14
- 14aR. Ziessel, G. Ulrich, A. Harriman, New J. Chem. 2007, 31, 496;
- 14bA. Loudet, K. Burgess, Chem. Rev. 2007, 107, 4891;
- 14cG. Ulrich, R. Ziessel, A. Harriman, Angew. Chem. Int. Ed. 2008, 47, 1184; Angew. Chem. 2008, 120, 1202;
- 14dA. C. Benniston, G. Copley, Phys. Chem. Chem. Phys. 2009, 11, 4124;
- 14eN. Boens, V. Leen, W. Dehaen, Chem. Soc. Rev. 2012, 41, 1130;
- 14fL. N. Sobenina, O. V. Petrova, K. B. Petrushenko, I. A. Ushakov, A. I. Mikhaleva, R. Meallet-Renault, B. A. Trofimov, Eur. J. Org. Chem. 2013, 4107.