A Stable Heterocyclic Amino(phosphanylidene-σ4-phosphorane) Germylene
Correction(s) for this article
-
Corrigendum: A Stable Heterocyclic Amino(phosphanylidene-σ4-phosphorane) Germylene
- Natalia Del Rio,
- Antoine Baceiredo,
- Nathalie Saffon-Merceron,
- Daisuke Hashizume,
- Dennis Lutters,
- Thomas Müller,
- Tsuyoshi Kato,
- Volume 55Issue 51Angewandte Chemie International Edition
- pages: 15698-15698
- First Published online: December 12, 2016
Natalia Del Rio
Université de Toulouse, UPS, and CNRS, LHFA UMR 5069, 118 route de Narbonne, 31062 Toulouse cedex 9, France
Search for more papers by this authorCorresponding Author
Dr. Antoine Baceiredo
Université de Toulouse, UPS, and CNRS, LHFA UMR 5069, 118 route de Narbonne, 31062 Toulouse cedex 9, France
Search for more papers by this authorDr. Nathalie Saffon-Merceron
Université de Toulouse, UPS, ICT-FR2599, 118 route de Narbonne, 31062 Toulouse cedex 9, France
Search for more papers by this authorDr. Daisuke Hashizume
Materials Characterization Support Unit, RIKEN Center for Emergent Matter Science (CEMS), Wako, Saitama, 351-0198 Japan
Search for more papers by this authorM. Sc. Dennis Lutters
Institut für Chemie, Carl von Ossietzky Universität Oldenburg, Carl von Ossietzky-Strasse 9–11, 26111 Oldenburg, Germany
Search for more papers by this authorProf. Dr. Thomas Müller
Institut für Chemie, Carl von Ossietzky Universität Oldenburg, Carl von Ossietzky-Strasse 9–11, 26111 Oldenburg, Germany
Search for more papers by this authorCorresponding Author
Dr. Tsuyoshi Kato
Université de Toulouse, UPS, and CNRS, LHFA UMR 5069, 118 route de Narbonne, 31062 Toulouse cedex 9, France
Search for more papers by this authorNatalia Del Rio
Université de Toulouse, UPS, and CNRS, LHFA UMR 5069, 118 route de Narbonne, 31062 Toulouse cedex 9, France
Search for more papers by this authorCorresponding Author
Dr. Antoine Baceiredo
Université de Toulouse, UPS, and CNRS, LHFA UMR 5069, 118 route de Narbonne, 31062 Toulouse cedex 9, France
Search for more papers by this authorDr. Nathalie Saffon-Merceron
Université de Toulouse, UPS, ICT-FR2599, 118 route de Narbonne, 31062 Toulouse cedex 9, France
Search for more papers by this authorDr. Daisuke Hashizume
Materials Characterization Support Unit, RIKEN Center for Emergent Matter Science (CEMS), Wako, Saitama, 351-0198 Japan
Search for more papers by this authorM. Sc. Dennis Lutters
Institut für Chemie, Carl von Ossietzky Universität Oldenburg, Carl von Ossietzky-Strasse 9–11, 26111 Oldenburg, Germany
Search for more papers by this authorProf. Dr. Thomas Müller
Institut für Chemie, Carl von Ossietzky Universität Oldenburg, Carl von Ossietzky-Strasse 9–11, 26111 Oldenburg, Germany
Search for more papers by this authorCorresponding Author
Dr. Tsuyoshi Kato
Université de Toulouse, UPS, and CNRS, LHFA UMR 5069, 118 route de Narbonne, 31062 Toulouse cedex 9, France
Search for more papers by this authorGraphical Abstract
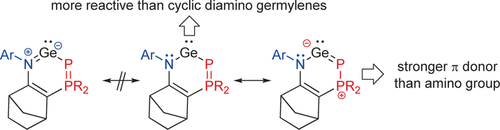
‘Germs’ of a different type: A stable heterocyclic germylene, in which the divalent germanium atom lies between a nitrogen atom and a phosphanylidene phosphorane group, was synthesized. Experimental and theoretical studies revealed the peculiar effect of the phosphanylidene phosphorane substituent, which presents π-donor abilities stronger than that of amino groups.
Abstract
A new stable heterocyclic germylene, in which the divalent germanium atom lies between a nitrogen atom and a phosphanylidene phosphorane group, was synthesized. Experimental and theoretical studies revealed the peculiar effect of phosphanylidene phosphorane substituent, which is a stronger π-donor towards germanium than an amino group is. Because of the weak phosphorus–germanium π-bond, this new germylene compound shows an enhanced reactivity compared to classical N-heterocyclic germylenes.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201511956-sup-0001-misc_information.pdf3.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Selected reviews on NHCs:
- 1aS. P. Nolan, N-Heterocyclic Carbenes in Synthesis, Wiley-VCH, Weinheim, 2006;
10.1002/9783527609451 Google Scholar
- 1bD. Martin, M. Melaimi, M. Soleilhavoup, G. Bertrand, Organometallics 2011, 30, 5304;
- 1cD. Enders, O. Niemeier, A. Henseler, Chem. Rev. 2007, 107, 5606;
- 1dN. Marion, S. Díez-González, S. P. Nolan, Angew. Chem. Int. Ed. 2007, 46, 2988; Angew. Chem. 2007, 119, 3046;
- 1eA. Grossmann, D. Enders, Angew. Chem. Int. Ed. 2012, 51, 314; Angew. Chem. 2012, 124, 320;
- 1fF. Glorius in N-Heterocyclic Carbenes in Transition Metal Catalysis, Vol. 21 (Ed.: ), Springer, Berlin, Heidelberg, 2006, p. 1;
- 1gT. Kato, E. Maerten, A. Baceiredo, in Transition Metal Complexes of Neutral η1-Carbon Ligands, Vol. 30, XI ed. ), Springer, Berlin, 2010, p. 131;
10.1007/978-3-642-04722-0_5 Google Scholar
- 1hS. Díez-González, S. P. Nolan, Coord. Chem. Rev. 2007, 251, 874.
- 2Reviews on stable carbenes:
- 2aW. Kirmse, Carbene Chemistry, 2nd ed., Academic Press, New York, 1971;
- 2bD. Bourissou, O. Guerret, F. P. Gabbai, G. Bertrand, Chem. Rev. 2000, 100, 39;
- 2cY. Canac, M. Soleilhavoup, S. Conejero, G. Bertrand, J. Organomet. Chem. 2004, 689, 3857;
- 2dO. Schuster, L. Yang, H. G. Raubenheimer, M. Albrecht, Chem. Rev. 2009, 109, 3445.
- 3Reviews on stable heavier analogues of carbenes:
- 3aY. Mizuhata, T. Sasamori, N. Tokitoh, Chem. Rev. 2009, 109, 3479;
- 3bM. Asay, C. Jones, M. Driess, Chem. Rev. 2011, 111, 354;
- 3cN. Tokitoh, R. Okazaki, Coord. Chem. Rev. 2000, 210, 251;
- 3dS. K. Mandala, H. W. Roesky, Chem. Commun. 2010, 46, 6016;
- 3eS. Nagendran, H. W. Roesky, Organometallics 2008, 27, 457.
- 4M. Driess, H. Grützmacher, Angew. Chem. Int. Ed. Engl. 1996, 35, 828; Angew. Chem. 1996, 108, 900.
- 5B. D. Rekken, T. M. Brown, J. C. Fettinger, H. M. Tuononen, P. P. Power, J. Am. Chem. Soc. 2012, 134, 6504.
- 6B. D. Rekken, T. M. Brown, J. C. Fettinger, F. Lips, H. M. Tuononen, R. H. Herber, P. P. Power, J. Am. Chem. Soc. 2013, 135, 10134.
- 7Phosphinocarbenes: J. Vignolle, X. Cattoën, D. Bourissou, Chem. Rev. 2009, 109, 3333.
- 8K. Izod, D. G. Rayner, S. M. El-Hamruni, R. W. Harrington, U. Baisch, Angew. Chem. Int. Ed. 2014, 53, 3636; Angew. Chem. 2014, 126, 3710.
- 9Ylide-stabilized carbenes:
- 9aF. Lavigne, A. El Kazzi, Y. Escudie, E. Maerten, T. Kato, N. Saffon-Merceron, V. Branchadell, F. P. Cosso, A. Baceiredo, Chem. Eur. J. 2014, 20, 12528;
- 9bM. Asay, T. Kato, N. Saffon-Merceron, F. Cossio, A. Baceiredo, G. Bertrand, Angew. Chem. Int. Ed. 2008, 47, 7530; Angew. Chem. 2008, 120, 7640.
- 10Ylide-stabilized silylene: M. Asay, S. Inoue, M. Driess, Angew. Chem. Int. Ed. 2011, 50, 9589; Angew. Chem. 2011, 123, 9763.
- 11Ylide-stabilized germylene: J. Berthe, J. M. Garcia, E. Ocando, T. Kato, N. Saffon-Merceron, A. De Cózar, F. P. Cossío, A. Baceiredo, J. Am. Chem. Soc. 2011, 133, 15930.
- 12
- 12aS. Shah, J. D. Protasiewicz, Chem. Commun. 1998, 1585;
- 12bS. Shah, G. P. A. Yap, J. D. Protasiewicz, J. Organomet. Chem. 2000, 608, 12.
- 13
- 13aR. Armbrust, M. Sanchez, R. Réau, U. Bergstrasser, M. Regitz, G. Bertrand, J. Am. Chem. Soc. 1995, 117, 10785;
- 13bM. Sanchez, R. Réau, F. Dahan, M. Regitz, G. Bertrand, Angew. Chem. Int. Ed. Engl. 1996, 35, 2228;
- 13cM. Sanchez, R. Réau, H. Gornitzka, F. Dahan, M. Regitz, G. Bertrand, J. Am. Chem. Soc. 1997, 119, 9720.
- 14
- 14aB. A. Surgenor, M. Bühl, A. M. Z. Slawin, J. D. Woollins, P. Kilian, Angew. Chem. Int. Ed. 2012, 51, 10150; Angew. Chem. 2012, 124, 10297;
- 14bB. A. Surgenor, B. A. Chalmers, K. S. A. Arachchige, A. M. Z. Slawin, J. D. Woollins, M. Bühl, P. Kilian, Inorg. Chem. 2014, 53, 6856.
- 15D. V. Partyka, M. P. Washington, J. B. Updegraff III, R. A. Woloszynek, J. D. Protasiewicz, Angew. Chem. Int. Ed. 2008, 47, 7489; Angew. Chem. 2008, 120, 7599.
- 16As a similar case, the use of NHC supported phosphinidenes as a π-donating substituent for the synthesis of stable phosphonium cation and its radical has been recently reported: A. M. Tondreau, Z. Benk, J. R. Harmer, H. Grützmacher, Chem. Sci. 2014, 5, 1545.
- 17O. Kühl, Coord. Chem. Rev. 2004, 248, 411.
- 18The phosphanylphosphaketene has been used as formal phosphino-phosphinidene source: Z. Li, X. Chen, M Bergeler, M. Reiher, C.-Y. Sua, H. Grützmacher, Dalton Trans. 2015, 44, 6431.
- 19
- 19aF. F. Puschmann, D. Stein, D. Heift, C. Hendriksen, Z. A. Gal, H.-F. Grützmacher, H. Grützmacher, Angew. Chem. Int. Ed. 2011, 50, 8420; Angew. Chem. 2011, 123, 8570;
- 19bD. Heift, Z. Benkő, H. Grützmacher, Dalton Trans. 2014, 43, 831;
- 19cG. Becker, W. Schwarz, N. Seidler, M. Westerhausen, Z. Anorg. Allg. Chem. 1992, 612, 72.
- 20CCDC 1443603 (2), 1435532 (3), 1443604 (4), 1435533 (11), and 1435534 (12) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 21J. M. García, E. Ocando-Mavarez, T. Kato, D. S. Coll, A. Briceño, N. Saffon-Merceron, A. Baceiredo, Inorg. Chem. 2012, 51, 8187.
- 22
- 22aJ. Escudié, C. Couret, J. Satgé, M. Andrianarison, J.-D. Andriamizaka, J. Am. Chem. Soc. 1985, 107, 3378;
- 22bM. Dräger, J. Escudié, C. Couret, H. Ranaivonjatovo, J. Satgé, Organometallics 1988, 7, 1010;
- 22cH. Ranaivonjatovo, J. Escudié, C. Couret, J. Satgé, M. Dräger, New J. Chem. 1989, 13, 389;
- 22dV. Y. Lee, M. Kawai, A. Sekiguchi, H. Ranaivonjatovo, J. Escudié, Organometallics 2009, 28, 4262.
- 23The presence of the phosphanylidene-σ4-phosphorane fragment in the derivatives 10–12 is clearly indicated by the high-field chemical shifts observed for the dicoordinate P in the 31P NMR spectroscopy. (δ=−211.1 ppm for 10, δ=−189.1 ppm for 11 and δ=−175.7 ppm for 12).
- 24K. Izod, Coord. Chem. Rev. 2012, 256, 2972.
- 25W. A. Herrmann, M. Denk, J. Behm, W. Scherer, F.-R. Klingan, H. Bock, B. Solouki, M. Wagner, Angew. Chem. Int. Ed. Engl. 1992, 31, 1485; Angew. Chem. 1992, 104, 1489.
- 26R. Rodriguez, T. Troadec, T. Kato, N. Saffon-Merceron, J.-M. Sotiropoulos, A. Baceiredo, Angew. Chem. Int. Ed. 2012, 51, 7158; Angew. Chem. 2012, 124, 7270.
- 27
- 27aR. Rodriguez, D. Gau, T. Troadec, N. Saffon-Merceron, V. Branchadell, A. Baceiredo, T. Kato, Angew. Chem. Int. Ed. 2013, 52, 8980; Angew. Chem. 2013, 125, 9150;
- 27bN. Nakata, R. Rodriguez, T. Troadec, N. Saffon-Merceron, J.-M. Sotiropoulos, A. Baceiredo, T. Kato, Angew. Chem. Int. Ed. 2013, 52, 10840; Angew. Chem. 2013, 125, 11040;
- 27cR. Rodriguez, T. Troadec, D. Gau, N. Saffon-Merceron, D. Hashizume, K. Miqueu, J.-M. Sotiropoulos, A. Baceiredo, T. Kato, Angew. Chem. Int. Ed. 2013, 52, 4426; Angew. Chem. 2013, 125, 4522.
- 28A. Jana, I. Omlor, V. Huch, H. S. Rzepa, D. Scheschkewitz, Angew. Chem. Int. Ed. 2014, 53, 9953; Angew. Chem. 2014, 126, 10112.
- 29For some examples of the dispersion interaction of divalent species. Dimerization of plumbylenes: H. Arp, J. Baumgartner, C. Marschner, P. Zark, T. Müller, J. Am. Chem. Soc. 2012, 134, 6409; stannylene and plumbylene complex of group 4 metallocenes: H. Arp, J. Baumgartner, C. Marschner, P. Zark, T. Müller, J. Am. Chem. Soc. 2012, 134, 10864; dimerization of stannylenes: J.-D. Guo, D. J. Liptot, S. Nagase, P. P. Power, Chem. Sci. 2015, 6, 6235.
- 30For a recent review on London dispersion interactions in chemistry, see: J. P. Wagner, P. Schreiner, Angew. Chem. Int. Ed. 2015, 54, 12274; Angew. Chem. 2015, 127, 12446.
- 31In the case of a model compound with small substituents, Ge–Ge distances are calculated to be much longer than those in 4. (see the Supporting Information).
- 32All quantum mechanical calculations were done using the Gaussian 09 program.
- 33Details of the computation are provided in the Supporting Information.
- 34K. B. Wiberg, Tetrahedron 1968, 24, 1083.
- 35See the Supporting Information for the bond lengths and WBIs of other related compounds.
- 36J. Pfeiffer, W. Maringgele, M. Noltemeyer, A. Meller, Chem. Ber. 1989, 122, 245.