Weak and Transient Protein Interactions Determined by Solid-State NMR
Dr. Hugh R. W. Dannatt
Centre de RMN à Très Hauts Champs—, Université de Lyon, Institut de Sciences Analytiques (CNRS/ ENS-Lyon/ UCB Lyon 1), 69100 Villeurbanne, France
Search for more papers by this authorMichele Felletti
Centre de RMN à Très Hauts Champs—, Université de Lyon, Institut de Sciences Analytiques (CNRS/ ENS-Lyon/ UCB Lyon 1), 69100 Villeurbanne, France
Search for more papers by this authorDr. Stefan Jehle
Centre de RMN à Très Hauts Champs—, Université de Lyon, Institut de Sciences Analytiques (CNRS/ ENS-Lyon/ UCB Lyon 1), 69100 Villeurbanne, France
Search for more papers by this authorYao Wang
Centre for Medical and Molecular Bioscience, School of Chemistry, University of Wollongong, Wollongong, New South Wales, 2522 Australia
Search for more papers by this authorProf. Lyndon Emsley
Centre de RMN à Très Hauts Champs—, Université de Lyon, Institut de Sciences Analytiques (CNRS/ ENS-Lyon/ UCB Lyon 1), 69100 Villeurbanne, France
Institut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne, 1015 Lausanne, Switzerland
Search for more papers by this authorProf. Nicholas E. Dixon
Centre for Medical and Molecular Bioscience, School of Chemistry, University of Wollongong, Wollongong, New South Wales, 2522 Australia
Search for more papers by this authorDr. Anne Lesage
Centre de RMN à Très Hauts Champs—, Université de Lyon, Institut de Sciences Analytiques (CNRS/ ENS-Lyon/ UCB Lyon 1), 69100 Villeurbanne, France
Search for more papers by this authorCorresponding Author
Dr. Guido Pintacuda
Centre de RMN à Très Hauts Champs—, Université de Lyon, Institut de Sciences Analytiques (CNRS/ ENS-Lyon/ UCB Lyon 1), 69100 Villeurbanne, France
Search for more papers by this authorDr. Hugh R. W. Dannatt
Centre de RMN à Très Hauts Champs—, Université de Lyon, Institut de Sciences Analytiques (CNRS/ ENS-Lyon/ UCB Lyon 1), 69100 Villeurbanne, France
Search for more papers by this authorMichele Felletti
Centre de RMN à Très Hauts Champs—, Université de Lyon, Institut de Sciences Analytiques (CNRS/ ENS-Lyon/ UCB Lyon 1), 69100 Villeurbanne, France
Search for more papers by this authorDr. Stefan Jehle
Centre de RMN à Très Hauts Champs—, Université de Lyon, Institut de Sciences Analytiques (CNRS/ ENS-Lyon/ UCB Lyon 1), 69100 Villeurbanne, France
Search for more papers by this authorYao Wang
Centre for Medical and Molecular Bioscience, School of Chemistry, University of Wollongong, Wollongong, New South Wales, 2522 Australia
Search for more papers by this authorProf. Lyndon Emsley
Centre de RMN à Très Hauts Champs—, Université de Lyon, Institut de Sciences Analytiques (CNRS/ ENS-Lyon/ UCB Lyon 1), 69100 Villeurbanne, France
Institut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne, 1015 Lausanne, Switzerland
Search for more papers by this authorProf. Nicholas E. Dixon
Centre for Medical and Molecular Bioscience, School of Chemistry, University of Wollongong, Wollongong, New South Wales, 2522 Australia
Search for more papers by this authorDr. Anne Lesage
Centre de RMN à Très Hauts Champs—, Université de Lyon, Institut de Sciences Analytiques (CNRS/ ENS-Lyon/ UCB Lyon 1), 69100 Villeurbanne, France
Search for more papers by this authorCorresponding Author
Dr. Guido Pintacuda
Centre de RMN à Très Hauts Champs—, Université de Lyon, Institut de Sciences Analytiques (CNRS/ ENS-Lyon/ UCB Lyon 1), 69100 Villeurbanne, France
Search for more papers by this authorGraphical Abstract
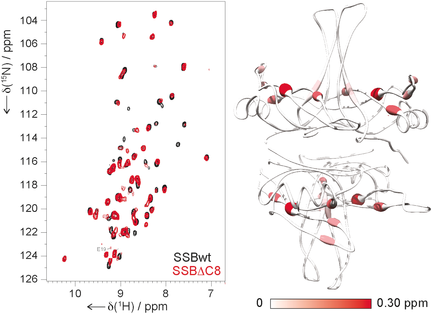
In a spin: A solid-state NMR approach based on high magnetic fields, fast magic-angle spinning, and deuteration was used to provide chemical-shift and relaxation mapping for characterizing the transient association between two regions in a 80 kDa protein assembly, the homotetrameric ssDNA-binding protein (SSB). Comparison of the wildtype (wt) and the truncated mutant SSBΔCt led to direct verification of a mechanism of regulation of E. coli DNA metabolism.
Abstract
Despite their roles in controlling many cellular processes, weak and transient interactions between large structured macromolecules and disordered protein segments cannot currently be characterized at atomic resolution by X-ray crystallography or solution NMR. Solid-state NMR does not suffer from the molecular size limitations affecting solution NMR, and it can be applied to molecules in different aggregation states, including non-crystalline precipitates and sediments. A solid-state NMR approach based on high magnetic fields, fast magic-angle sample spinning, and deuteration provides chemical-shift and relaxation mapping that enabled the characterization of the structure and dynamics of the transient association between two regions in an 80 kDa protein assembly. This led to direct verification of a mechanism of regulation of E. coli DNA metabolism.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201511609-sup-0001-misc_information.pdf1.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1P. Tompa, M. Fuxreiter, Trends Biochem. Sci. 2008, 33, 2–8.
- 2P. E. Wright, H. J. Dyson, Nat. Rev. Mol. Cell Biol. 2015, 16, 18–29.
- 3
- 3aA. N. Volkov, J. A. R. Worrall, E. Holtzmann, M. Ubbink, Proc. Natl. Acad. Sci. USA 2006, 103, 18945–18950;
- 3bG. M. Clore, J. Iwahara, Chem. Rev. 2009, 109, 4108–4139.
- 4L. Emsley, I. Bertini, Acc. Chem. Res. 2012, 46, 1912–1913.
- 5
- 5aI. Bertini, C. Luchinat, G. Parigi, E. Ravera, B. Reif, P. Turano, Proc. Natl. Acad. Sci. USA 2011, 108, 10396–10399;
- 5bC. Gardiennet, A. K. Schuetz, A. Hunkeler, B. Kunert, L. Terradot, A. Boeckmann, B. H. Meier, Angew. Chem. Int. Ed. 2012, 51, 7855–7858; Angew. Chem. 2012, 124, 7977–7980.
- 6J. R. Lewandowski, M. E. Halse, M. Blackledge, L. Emsley, Science 2015, 348, 578–581.
- 7
- 7aL. B. Andreas, T. Le Marchand, K. Jaudzems, G. Pintacuda, J. Magn. Reson. 2015, 253, 36–49;
- 7bA. Boeckmann, M. Ernst, B. H. Meier, J. Magn. Reson. 2015, 253, 71–79.
- 8
- 8aM. J. Knight, et al., Angew. Chem. Int. Ed. 2011, 50, 11697–11701; Angew. Chem. 2011, 123, 11901–11905;
- 8bA. J. Nieuwkoop, W. T. Franks, K. Rehbein, A. Diehl, U. Akbey, F. Engelke, L. Emsley, G. Pintacuda, H. Oschkinat, J. Biomol. NMR 2015, 61, 161–171.
- 9
- 9aE. Barbet-Massin, et al., J. Am. Chem. Soc. 2014, 136, 12489–12497;
- 9bS. Q. Xiang, V. Chevelkov, S. Becker, A. Lange, J. Biomol. NMR 2014, 60, 85–90;
- 9cL. B. Andreas, et al., J. Biomol. NMR 2015, 62, 253–261;
- 9dS. Wang, et al., Chem. Commun. 2015, 51, 15055–15058.
- 10
- 10aM. Huber, S. Hiller, P. Schanda, M. Ernst, A. Boeckmann, R. Verel, B. H. Meier, ChemPhysChem 2011, 12, 915–918;
- 10bM. J. Knight, A. J. Pell, I. Bertini, I. C. Felli, L. Gonnelli, R. Pierattelli, T. Herrmann, L. Emsley, G. Pintacuda, Proc. Natl. Acad. Sci. USA 2012, 109, 11095–11100;
- 10cR. Linser, B. Bardiaux, L. B. Andreas, S. G. Hyberts, V. K. Morris, G. Pintacuda, M. Sunde, A. H. Kwan, G. Wagner, J. Am. Chem. Soc. 2014, 136, 11002–11010;
- 10dV. Agarwal, et al., Angew. Chem. Int. Ed. 2014, 53, 12253–12256; Angew. Chem. 2014, 126, 12450–12453.
- 11
- 11aM. Tollinger, A. C. Sivertsen, B. H. Meier, M. Ernst, P. Schanda, J. Am. Chem. Soc. 2012, 134, 14800–14807;
- 11bS. H. Park, C. Yang, S. J. Opella, L. J. Mueller, J. Magn. Reson. 2013, 237, 164–168;
- 11cP. Ma, J. D. Haller, J. Zajakala, P. Macek, A. C. Sivertsen, D. Willbold, J. Boisbouvier, P. Schanda, Angew. Chem. Int. Ed. 2014, 53, 4312–4317; Angew. Chem. 2014, 126, 4400–4405;
- 11dS. Asami, J. R. Porter, O. F. Lange, B. Reif, J. Am. Chem. Soc. 2015, 137, 1094–1100.
- 12
- 12aT. Sinnige, M. Daniels, M. Baldus, M. Weingarth, J. Am. Chem. Soc. 2014, 136, 4452–4455;
- 12bJ. M. Lamley, et al., J. Am. Chem. Soc. 2014, 136, 16800–16806;
- 12cP. Schanda, S. Triboulet, C. Laguri, C. M. Bougault, I. Ayala, M. Callon, M. Arthur, J.-P. Simorre, J. Am. Chem. Soc. 2014, 136, 17852–17860;
- 12dM. T. Eddy, Y. Su, R. Silvers, L. Andreas, L. Clark, G. Wagner, G. Pintacuda, L. Emsley, R. G. Griffin, J. Biomol. NMR 2015, 61, 299–310;
- 12eL. B. Andreas, M. Reese, M. T. Eddy, V. Gelev, Q. Z. Ni, E. A. Miller, L. Emsley, G. Pintacuda, J. J. Chou, R. G. Griffin, J. Am. Chem. Soc. 2015, in press;
- 12fE. Barbet-Massin, C.-T. Huang, V. Daebel, S.-T. D. Hsu, B. Reif, Angew. Chem. Int. Ed. 2015, 54, 4367–4369; Angew. Chem. 2015, 127, 4441–4444;
- 12gJ. M. Lamley, C. Öster, R. A. Stevens, J. R. Lewandowski, Angew. Chem. Int. Ed. 2015, 54, 15374–15378; Angew. Chem. 2015, 127, 15594—15598.
- 13
- 13aP. M. Schaeffer, M. J. Headlam, N. E. Dixon, IUBMB Life 2005, 57, 5–12;
- 13bC. S. McHenry, Annu. Rev. Biochem. 2011, 80, 403–436;
- 13cA. Robinson, R. J. Causer, N. E. Dixon, Curr. Drug Targets 2012, 13, 352–372.
- 14R. D. Shereda, A. G. Kozlov, T. M. Lohman, M. M. Cox, J. L. Keck, Crit. Rev. Biochem. Mol. Biol. 2008, 43, 289–318.
- 15R. R. Meyer, P. S. Laine, Microbiol. Rev. 1990, 54, 342–380.
- 16D. Shishmarev, Y. Wang, C. E. Mason, X. C. Su, A. J. Oakley, B. Graham, T. Huber, N. E. Dixon, G. Otting, Nucleic Acids Res. 2014, 42, 2750–2757.
- 17Z. Kelman, A. Yuzhakov, J. Andjelkovic, M. O'Donnell, EMBO J. 1998, 17, 2436–2449.
- 18
- 18aD. Lu, J. L. Keck, Proc. Natl. Acad. Sci. USA 2008, 105, 9169–9174;
- 18bE. Antony, E. Weiland, Q. Yuan, C. M. Manhart, N. Binh, A. G. Kozlov, C. S. McHenry, T. M. Lohman, J. Mol. Biol. 2013, 425, 4802–4819.
- 19
- 19aS. Raghunathan, C. S. Ricard, T. M. Lohman, G. Waksman, Proc. Natl. Acad. Sci. USA 1997, 94, 6652–6657;
- 19bS. Raghunathan, A. G. Kozlov, T. M. Lohman, G. Waksman, Nat. Struct. Biol. 2000, 7, 648–652.
- 20S. N. Savvides, S. Raghunathan, K. Futterer, A. G. Kozlov, T. M. Lohman, G. Waksman, Protein Sci. 2004, 13, 1942–1947.
- 21A. G. Kozlov, M. M. Cox, T. M. Lohman, J. Biol. Chem. 2010, 285, 17246–17252.
- 22A. G. Kozlov, J. M. Eggington, M. M. Cox, T. M. Lohman, Biochemistry 2010, 49, 8266–8275.
- 23C. E. Mason, S. Jergic, A. T. Y. Lo, Y. Wang, N. E. Dixon, J. L. Beck, J. Am. Soc. Mass Spectrom. 2013, 24, 274–285.
- 24A. Marchetti, et al., Angew. Chem. Int. Ed. 2012, 51, 10756–10759; Angew. Chem. 2012, 124, 10914–10917.
- 25I. Bertini, C. Luchinat, G. Parigi, E. Ravera, Acc. Chem. Res. 2013, 46, 2059–2069.
- 26M. P. Williamson, Prog. Nucl. Magn. Reson. Spectrosc. 2013, 73, 1–16.
- 27X.-C. Su, Y. Wang, H. Yagi, D. Shishmarev, C. E. Mason, P. J. Smith, M. Vandevenne, N. E. Dixon, G. Otting, Biochemistry 2014, 53, 1925–1934.