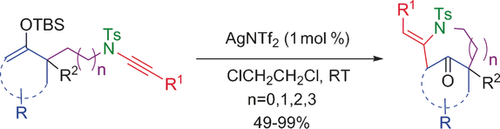
Silver-Catalyzed 7-exo-dig Cyclization of Silylenolether-ynesulfonamides
Dr. Clément F. Heinrich
Laboratoire de Chimie Organique Synthétique, Institut de Chimie, 1, rue Blaise Pascal, BP296/R8, 67008 Strasbourg, France
Search for more papers by this authorIndira Fabre
Département de Chimie, Ecole Normale Supérieure—PSL Research University, Sorbonne Universités—UPMC Univ Paris 06, CNRS UMR 8640 PASTEUR, 24, rue Lhomond, 75005 Paris, France
Search for more papers by this authorCorresponding Author
Dr. Laurence Miesch
Laboratoire de Chimie Organique Synthétique, Institut de Chimie, 1, rue Blaise Pascal, BP296/R8, 67008 Strasbourg, France
Search for more papers by this authorDr. Clément F. Heinrich
Laboratoire de Chimie Organique Synthétique, Institut de Chimie, 1, rue Blaise Pascal, BP296/R8, 67008 Strasbourg, France
Search for more papers by this authorIndira Fabre
Département de Chimie, Ecole Normale Supérieure—PSL Research University, Sorbonne Universités—UPMC Univ Paris 06, CNRS UMR 8640 PASTEUR, 24, rue Lhomond, 75005 Paris, France
Search for more papers by this authorCorresponding Author
Dr. Laurence Miesch
Laboratoire de Chimie Organique Synthétique, Institut de Chimie, 1, rue Blaise Pascal, BP296/R8, 67008 Strasbourg, France
Search for more papers by this authorGraphical Abstract
Dig this! Cyclization of silylenolether-ynesulfonamides proceeds at ambient temperature under mild reaction conditions under silver catalysis. Bridged compounds were obtained exclusively through 7-exo-dig reactions. The protocol is applicable to a wide range of substrates, thus leading to azabicyclic frameworks.
Abstract
Cyclization of silylenolether-ynesulfonamides proceeds at ambient temperature under mild reaction conditions under silver catalysis. Bridged compounds were obtained exclusively through 7-exo-dig reactions. The protocol is applicable to a wide range of substrates, thus leading to azabicyclic frameworks.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201510708-sup-0001-misc_information.pdf1.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews of ynamides, see:
- 1aK. A. DeKorver, H. Li, A. G. Lohse, R. Hayashi, Z. Lu, Y. Zhang, R. P. Hsung, Chem. Rev. 2010, 110, 5064;
- 1bG. Evano, A. Coste, K. Jouvin, Angew. Chem. Int. Ed. 2010, 49, 2840; Angew. Chem. 2010, 122, 2902;
- 1cG. Evano, K. Jouvin, A. Coste, Synthesis 2013, 17;
- 1dX. N. Wang, H. S. Yeom, L. C. Fang, S. He, Z. X. Ma, B. L. Kedrowski, R. P. Hsung, Acc. Chem. Res. 2014, 47, 560.
- 2For publications using platinum, see:
- 2aF. Marion, J. Coulomb, C. Courillon, L. Fensterbank, M. Malacria, Org. Lett. 2004, 6, 1509;
- 2bF. Marion, J. Coulomb, A. Servais, C. Courillon, L. Fensterbank, M. Malacria, Tetrahedron 2006, 62, 3856; For gold, see:
- 2cA. S. K. Hashmi, R. Salathé, W. Frey, Synlett 2007, 1763;
- 2dF. M. Istrate, A. K. Buzas, I. D. Jurberg, Y. Odabachian, F. Gagosz, Org. Lett. 2008, 10, 925;
- 2eA. S. K. Hashmi, M. Rudolph, J. W. Bats, W. Frey, F. Rominger, T. Oeser, Chem. Eur. J. 2008, 14, 6672;
- 2fS. Couty, C. Meyer, J. Cossy, Angew. Chem. Int. Ed. 2006, 45, 6726; Angew. Chem. 2006, 118, 6878;
- 2gC. W. Li, K. Pati, G. Y. Lin, S. M. Abu Sohel, H. H. Hung, R. S. Liu, Angew. Chem. Int. Ed. 2010, 49, 9891; Angew. Chem. 2010, 122, 10087;
- 2hN. Ghosh, S. Nayak, A. K. Sahoo, Chem. Eur. J. 2013, 19, 9428;
- 2iK. B. Wang, R. Q. Ran, S. D. Xiu, C. Y. Li, Org. Lett. 2013, 15, 2374;
- 2jM. C. Blanco Jaimes, V. Weingand, F. Rominger, A. S. K. Hashmi, Chem. Eur. J. 2013, 19, 12504;
- 2kH. V. Adcock, T. Langer, P. W. Davies, Chem. Eur. J. 2014, 20, 7262;
- 2lT. Wang, S. Shi, M. M. Hansmann, E. Rettenmeier, M. Rudolph, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2014, 53, 3715; Angew. Chem. 2014, 126, 3789;
- 2mJ. Liu, M. Chen, L. Zhang, Y. Liu, Chem. Eur. J. 2015, 21, 1009;
- 2nY. Tokimizu, M. Wieteck, M. Rudolph, S. Oishi, N. Fujii, A. S. K. Hashmi, H. Ohno, Org. Lett. 2015, 17, 604; for palladium, see:
- 2oP. Y. Yao, Y. Zhang, R. P. Hsung, K. Zhao, Org. Lett. 2008, 10, 4275;
- 2pK. Dooleweerdt, T. Ruhland, T. Skrydstrup, Org. Lett. 2009, 11, 221;
- 2qR. L. Greenaway, C. D. Campbell, O. T. Holton, C. A. Russell, E. A. Anderson, Chem. Eur. J. 2011, 17, 14366;
- 2rP. R. Walker, C. D. Campbell, A. Suleman, G. Carr, E. A. Anderson, Angew. Chem. Int. Ed. 2013, 52, 9139; Angew. Chem. 2013, 125, 9309;
- 2sG. Liu, W. Kong, J. Che, G. Zhu, Adv. Synth. Catal. 2014, 356, 3314; For ruthenium, see:
- 2tN. Saito, Y. Sato, M. Mori, Org. Lett. 2002, 4, 803;
- 2uJ. Huang, H. Xiong, R. P. Hsung, C. Rameshkumar, J. A. Mulder, T. P. Grebe, Org. Lett. 2002, 4, 2417;
- 2vM. Mori, H. Wakamatsu, N. Saito, Y. Sato, R. Narita, Y. Sato, R. Fujita, Tetrahedron 2006, 62, 3872;
- 2wH. Wakamatsu, M. Sakagami, M. Hanata, M. Takeshita, M. Mori, Macromol. Symp. 2010, 293, 5; For HNTf2, see:
- 2xY. Zhang, R. P. Hsung, X. Zhang, J. Huang, B. W. Slafer, A. Davis, Org. Lett. 2005, 7, 1047; for copper, see:
- 2yA. S. K. Hashmi, A. M. Schuster, M. Zimmer, F. Rominger, Chem. Eur. J. 2011, 17, 5511;
- 2zW. Gati, F. Couty, T. Boubaker, M. M. Rammah, M. B. Rammah, G. Evano, Org. Lett. 2013, 15, 3122; for rhodium, see:
- 2aaT. Nishimura, Y. Takigushi, Y. Maeda, T. Hayashi, Adv. Synth. Catal. 2013, 355, 1374;
- 2abR. Liu, G. N. Winston-McPherson, Z. Y. Yang, X. Zhou, W. Song, I. A. Guzei, X. Xu, W. Tang, J. Am. Chem. Soc. 2013, 135, 8201; for silver, see:
- 2acG. Y. Lin, C. W. Li, S. H. Hung, R. S. Liu, Org. Lett. 2008, 10, 5059;
- 2adP. Garcia, Y. Harrak, L. Diab, P. Cordier, C. Ollivier, V. Gandon, M. Malacria, L. Fensterbank, C. Aubert, Org. Lett. 2011, 13, 2952;
- 2aeT. Sueda, A. Kawada, Y. Urashi, N. Teno, Org. Lett. 2013, 15, 1560.
- 3
- 3aC. Ferrer, A. M. Echavarren, Angew. Chem. Int. Ed. 2006, 45, 1105; Angew. Chem. 2006, 118, 1123;
- 3bC. Ferrer, C. H. M. Amijs, A. M. Echavarren, Chem. Eur. J. 2007, 13, 1358;
- 3cK. Wilckens, M. Uhlemann, C. Czekelius, Chem. Eur. J. 2009, 15, 13323;
- 3dH. Ito, H. Ohmiya, M. Sawamura, Org. Lett. 2010, 12, 4380;
- 3eT. Iwai, H. Okochi, H. Ito, M. Sawamura, Angew. Chem. Int. Ed. 2013, 52, 4239; Angew. Chem. 2013, 125, 4333;
- 3fD. Pflästerer, E. Rettenmeier, S. Schneider, E. de Las Heras Ruiz, M. Rudoplh, A. S. K. Hashmi, Chem. Eur. J. 2014, 20, 6752;
- 3gD. Pflästerer, S. Schumacher, M. Rudolph, A. S. K. Hashmi, Chem. Eur. J. 2015, 21, 11585.
- 4C. Schäfer, M. Miesch, L. Miesch, Chem. Eur. J. 2012, 18, 8028.
- 5L. Miesch, T. Welsch, V. Rietsch, M. Miesch, Chem. Eur. J. 2009, 15, 4394.
- 6
- 6aD. Enders, M. Voith, S. J. Ince, Synthesis 2002, 1775;
- 6bX. E. Hu, Tetrahedron 2004, 60, 2701.
- 7Y. Zhang, R. P. Hsung, M. R. Tracey, K. C. M. Kurtz, E. L. Vera, Org. Lett. 2004, 6, 1151.
- 8Less hindered kinetic versus thermodynamic TMS-silyloxy ene-ynesulfonamides were obtained in a ratio 1.2:1, thus leading to bridged the keto-ynesulfonamide in far lower yield.
- 9An X-ray structural determination of the latter clearly showed the generation of a single silyl enol ether. CCDC 1415013 (4 a) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 10DFT calculations support experimental results observed. Refer to the Supporting Information for details.
- 11Kinetics studies using AgNTf2 and [Au(PPh3)(NTf2)] have shown similar profiles for both catalysts, although the gold catalyst was faster than silver catalysis (t
 =2 min for [Au(PPh3)(NTf2)] and t
=2 min for [Au(PPh3)(NTf2)] and t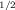 =5 min for AgNTf2).
=5 min for AgNTf2).
- 12CCDC 1415014 (5 a) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 137-endo-dig cyclization of keteneiminium cation is proposed although the 7-exo-dig compound is obtained.
- 14CCDC 1415015 (5 s) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 15CCDC 1415016 (5 y) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 16
- 16aA. Buzas, F. Gagosz, Org. Lett. 2006, 8, 515;
- 16bA. K. Buzas, F. M. Istrate, F. Gagosz, Tetrahedron 2009, 65, 1889;
- 16cA. S. K. Hashmi, T. Dondeti Ramamurthi, F. Rominger, J. Organomet. Chem. 2009, 694, 592;
- 16dT. Wang, S. Shi, M. Rudolph, A. S. K. Hashmi, Adv. Synth. Catal. 2014, 356, 2337.
- 17CCDC 1415017 (7) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 18A. S. K. Hashmi, Angew. Chem. Int. Ed. 2010, 49, 5232; Angew. Chem. 2010, 122, 5360.
- 19
- 19aA. Szadkowska, K. Zukowska, A. E. Pazio, K. Wozniak, R. Kadyrov, K. Greta, Organometallics 2011, 30, 1130;
- 19bA. Tanakit, M. Rouffet, D. P. Martin, S. M. Cohen, Dalton Trans. 2012, 41, 6507.
- 20D. R. Whitcomb, M. Rajeswaran, J. Chem. Crystallogr. 2006, 36, 587.