Reaction of Diazo Compounds with Difluorocarbene: An Efficient Approach towards 1,1-Difluoroolefins
Zhikun Zhang
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871 (China)
Search for more papers by this authorWeizhi Yu
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871 (China)
Search for more papers by this authorChenggui Wu
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871 (China)
Search for more papers by this authorChengpeng Wang
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871 (China)
Search for more papers by this authorYan Zhang
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jianbo Wang
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871 (China)
The State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032 (China)
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871 (China)Search for more papers by this authorZhikun Zhang
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871 (China)
Search for more papers by this authorWeizhi Yu
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871 (China)
Search for more papers by this authorChenggui Wu
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871 (China)
Search for more papers by this authorChengpeng Wang
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871 (China)
Search for more papers by this authorYan Zhang
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jianbo Wang
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871 (China)
The State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032 (China)
Beijing National Laboratory of Molecular Sciences (BNLMS) and Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking University, Beijing 100871 (China)Search for more papers by this authorGraphical Abstract
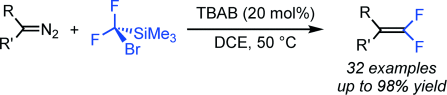
Formal carbene dimerization: The difluoromethylenation of diazo compounds was achieved under mild conditions with TMSCF2Br as the difluoromethylene source and tetrabutylammonium bromide (TBAB) as the initiator. The chemoselective formal carbene dimerization is achieved owing to the electronic properties and the relative stability of the difluorocarbene intermediate.
Abstract
A transition-metal-free difluoromethylenation of diazo compounds that proceeds under mild conditions has been developed and is based on the use of TMSCF2Br as the difluoromethylene source and tetrabutylammonium bromide (TBAB) as the promoter. The chemoselective formal carbene dimerization reaction is achieved owing to the electronic properties and the relative stability of the difluorocarbene intermediate.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201509711_sm_miscellaneous_information.pdf1.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see:
- 1aT. Ye, M. A. McKervey, Chem. Rev. 1994, 94, 1091–1160;
- 1bM. P. Doyle, M. A. McKervey, T. Ye in Modern Catalytic Methods for Organic Synthesis with Diazo Compounds, Wiley, New York, 1998;
- 1cM. P. Doyle, D. C. Forbes, Chem. Rev. 1998, 98, 911–935;
- 1dH. M. L. Davies, J. R. Manning, Nature 2008, 451, 417–424;
- 1eZ. Zhang, J. Wang, Tetrahedron 2008, 64, 6577–6605;
- 1fY. Zhang, J. Wang, Chem. Commun. 2009, 5350–5361;
- 1gX. Zhao, Y. Zhang, J. Wang, Chem. Commun. 2012, 48, 10162–10173;
- 1hH. M. L. Davies, Y. Lian, Acc. Chem. Res. 2012, 45, 923–935;
- 1iX. Guo, W. Hu, Acc. Chem. Res. 2013, 46, 2427–2440;
- 1jT. Hashimoto, K. Maruoka, Bull. Chem. Soc. Jpn. 2013, 86, 1217–1230;
- 1kG. K. Murphy, C. Stewart, F. G. West, Tetrahedron 2013, 69, 2667–2686;
- 1lA. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire, M. A. McKervey, Chem. Rev. 2015, 115, 9981–10080;
- 1mW. Kirmse in Advances in Carbene Chemistry, Vol. 3 (Ed.: ), Elsevier, Amsterdam, 2001, pp. 1–52.
10.1016/S1079-350X(01)80003-4 Google Scholar
- 2For selected examples of the homocoupling of diazo substrates, see:
- 2aW. Baratta, A. Del Zotto, P. Rigo, Chem. Commun. 1997, 2163–2164;
- 2bA. Del Zotto, W. Baratta, G. Verardo, P. Rigo, Eur. J. Org. Chem. 2000, 2795–2801;
- 2cM. P. Doyle, M. Yan, J. Org. Chem. 2002, 67, 602–604;
- 2dD. M. Hodgson, D. Angrish, Chem. Commun. 2005, 4902–4904;
- 2eD. M. Hodgson, D. Angrish, J. Mol. Catal. A 2006, 254, 93–95;
- 2fD. M. Hodgson, D. Angrish, Chem. Eur. J. 2007, 13, 3470–3479.
- 3For selected examples of intramolecular couplings of bis(diazo) substrates, see:
- 3aJ. Font, F. Serratosa, J. Valls, Chem. Commun. 1970, 721–722;
- 3bS. Kulkowit, M. A. McKervey, J. Chem. Soc. Chem. Commun. 1978, 1069–1070;
- 3cM. P. Doyle, W. Hu, I. M. Phillips, Org. Lett. 2000, 2, 1777–1779;
- 3dG.-Y. Li, C.-M. Che, Org. Lett. 2004, 6, 1621–1623.
- 4Y. Xia, Z. Liu, Q. Xiao, P. Qu, R. Ge, Y. Zhang, J. Wang, Angew. Chem. Int. Ed. 2012, 51, 5714–5717; Angew. Chem. 2012, 124, 5812–5815.
- 5For cross-couplings of diazo compounds, see:
- 5aJ. H. Hansen, B. T. Parr, P. Pelphrey, Q. Jin, J. Autschbach, H. M. L. Davies, Angew. Chem. Int. Ed. 2011, 50, 2544–2548; Angew. Chem. 2011, 123, 2592–2596;
- 5bD. Zhang, G. Xu, D. Ding, C. Zhu, J. Li, J. Sun, Angew. Chem. Int. Ed. 2014, 53, 11070–11074; Angew. Chem. 2014, 126, 11250–11254;
- 5cC. Zhu, G. Xu, K. Liu, L. Qiu, S. Peng, J. Sun, Chem. Commun. 2015, 51, 12768–12770;
- 5dC. Zhu, G. Xu, D. Ding, J. Sun, Org. Lett. 2015, 17, 4244–4247;
- 5eC. Zhu, L. Qiu, G. Xu, J. Li, J. Sun, Chem. Eur. J. 2015, 21, 12871–12875.
- 6For a report on carbene dimerization from propargylic esters and diazo compounds, see: C. Vovard-Le Bray, S. Dérien, P. H. Dixneuf, Angew. Chem. Int. Ed. 2009, 48, 1439–1442; Angew. Chem. 2009, 121, 1467–1470.
- 7F. Hu, J. Yang, Y. Xia, C. Ma, H. Xia, Y. Zhang, J. Wang, Org. Chem. Front. 2015, 2, 1450–1456.
- 8For selected reviews on difluorocarbene, see:
- 8aQ.-Y. Chen, J. Fluorine Chem. 1995, 72, 241–246;
- 8bD. L. S. Brahms, W. P. Dailey, Chem. Rev. 1996, 96, 1585–1632;
- 8cT. Taguchi, M. Okada, J. Fluorine Chem. 2000, 105, 279–283;
- 8dW. R. Dolbier, Jr., M. A. Battiste, Chem. Rev. 2003, 103, 1071–1098;
- 8eB. Gao, C. Ni, J. Hu, Chimia 2014, 68, 414–418;
- 8fC.-P. Zhang, Q.-Y. Chen, Y. Guo, J.-C. Xiao, Y.-C. Gu, Coord. Chem. Rev. 2014, 261, 28–72;
- 8gX. Shen, J. Hu, Eur. J. Org. Chem. 2014, 4437–4451;
- 8hC. Ni, J. Hu, Synthesis 2014, 842–863;
- 8iC. Ni, M. Hu, J. Hu, Chem. Rev. 2015, 115, 765–825; for related recent reports on fluorinated diazo compounds or carbenoids, see:
- 8jP. K. Mykhailiuk, Angew. Chem. Int. Ed. 2015, 54, 6558–6561; Angew. Chem. 2015, 127, 6658–6661;
- 8kE. Emer, J. Twilton, M. Tredwell, S. Calderwood, T. L. Collier, B. Liegault, M. Taillefer, V. Gouverneur, Org. Lett. 2014, 16, 6004–6007;
- 8lB. Morandi, E. M. Carreira, Angew. Chem. Int. Ed. 2010, 49, 938–941; Angew. Chem. 2010, 122, 950–953.
- 9For selected reviews on Fischer carbene complexes, see:
- 9aW. D. Wulff in Comprehensive Organometallic Chemistry II, Vol. 12 (Eds.: ), Pergamon, Oxford, UK, 1995, p. 470;
- 9bL. S. Hegedus in Comprehensive Organometallic Chemistry II, Vol. 12 (Eds.: ), Pergamon, Oxford, UK, 1995, p. 549;
- 9cD. F. Harvey, D. M. Sigano, Chem. Rev. 1996, 96, 271–288;
- 9dM. A. Sierra, Chem. Rev. 2000, 100, 3591–3638;
- 9eA. de Meijere, H. Schirmer, M. Duetsch, Angew. Chem. Int. Ed. 2000, 39, 3964–4002;
10.1002/1521-3773(20001117)39:22<3964::AID-ANIE3964>3.0.CO;2-C CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 4124–4162;
- 9fJ. Barluenga, J. Santamaría, M. Tomás, Chem. Rev. 2004, 104, 2259–2284;
- 9gK. H. Dötz, J. Stendel, Chem. Rev. 2009, 109, 3227–3274;
- 9hI. Fernández, F. P. Cossío, M. A. Sierra, Acc. Chem. Res. 2011, 44, 479–490.
- 10
- 10aM. Hu, Z. He, B. Gao, L. Li, C. Ni, J. Hu, J. Am. Chem. Soc. 2013, 135, 17302–17305;
- 10bB. Gao, Y. Zhao, M. Hu, C. Ni, J. Hu, Chem. Eur. J. 2014, 20, 7803–7810, and references therein.
- 11
- 11aF. Wang, W. Zhang, J. Zhu, H. Li, K.-W. Huang, J. Hu, Chem. Commun. 2011, 47, 2411–2413;
- 11bM. D. Kosobokov, A. D. Dilman, V. V. Levin, M. I. Struchkova, J. Org. Chem. 2012, 77, 5850–5855;
- 11cV. V. Levin, A. A. Zemtsov, M. I. Struchkova, A. D. Dilman, Org. Lett. 2013, 15, 917–919;
- 11dL. Li, F. Wang, C. Ni, J. Hu, Angew. Chem. Int. Ed. 2013, 52, 12390–12394; Angew. Chem. 2013, 125, 12616–12620;
- 11eK. Aikawa, W. Toya, Y. Nakamura, K. Mikami, Org. Lett. 2015, 17, 4996–4999.
- 12F. Ye, C. Wang, Y. Zhang, J. Wang, Angew. Chem. Int. Ed. 2014, 53, 11625–11628; Angew. Chem. 2014, 126, 11809–11812, and references therein.
- 13For a recent review on N-tosylhydrazones in organic synthesis, see: Y. Zhang, J. Wang in Topics in Current Chemistry, Stereoselective Alkene Synthesis (Ed: ), Springer, Heidelberg, 2012, 327, 239–270.
10.1007/128_2012_322 Google Scholar
- 14While the manuscript was under review, a related process was published; see: M. Hu, C. Ni, L. Li, Y. Han, J. Hu, J. Am. Chem. Soc. 2015, 137, DOI: 10.1021/jacs.5b09888.