Measurement of Local Sodium Ion Levels near Micelle Surfaces with Fluorescent Photoinduced-Electron-Transfer Sensors
Corresponding Author
Dr. Seiichi Uchiyama
School of Chemistry and Chemical Engineering, Queen's University, Belfast BT9 5AG (Northern Ireland)
Graduate School of Pharmaceutical Sciences, The University of Tokyo, 7-3-1 Hongo Bunkyo-ku Tokyo 113-0033 (Japan)
School of Chemistry and Chemical Engineering, Queen's University, Belfast BT9 5AG (Northern Ireland)Search for more papers by this authorEiko Fukatsu
Graduate School of Pharmaceutical Sciences, The University of Tokyo, 7-3-1 Hongo Bunkyo-ku Tokyo 113-0033 (Japan)
Search for more papers by this authorDr. Gareth D. McClean
School of Chemistry and Chemical Engineering, Queen's University, Belfast BT9 5AG (Northern Ireland)
Search for more papers by this authorCorresponding Author
Prof. A. Prasanna de Silva
School of Chemistry and Chemical Engineering, Queen's University, Belfast BT9 5AG (Northern Ireland)
School of Chemistry and Chemical Engineering, Queen's University, Belfast BT9 5AG (Northern Ireland)Search for more papers by this authorCorresponding Author
Dr. Seiichi Uchiyama
School of Chemistry and Chemical Engineering, Queen's University, Belfast BT9 5AG (Northern Ireland)
Graduate School of Pharmaceutical Sciences, The University of Tokyo, 7-3-1 Hongo Bunkyo-ku Tokyo 113-0033 (Japan)
School of Chemistry and Chemical Engineering, Queen's University, Belfast BT9 5AG (Northern Ireland)Search for more papers by this authorEiko Fukatsu
Graduate School of Pharmaceutical Sciences, The University of Tokyo, 7-3-1 Hongo Bunkyo-ku Tokyo 113-0033 (Japan)
Search for more papers by this authorDr. Gareth D. McClean
School of Chemistry and Chemical Engineering, Queen's University, Belfast BT9 5AG (Northern Ireland)
Search for more papers by this authorCorresponding Author
Prof. A. Prasanna de Silva
School of Chemistry and Chemical Engineering, Queen's University, Belfast BT9 5AG (Northern Ireland)
School of Chemistry and Chemical Engineering, Queen's University, Belfast BT9 5AG (Northern Ireland)Search for more papers by this authorGraphical Abstract
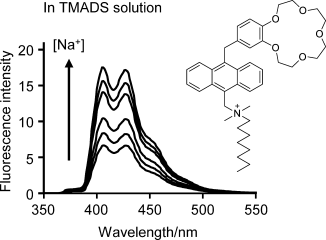
The local Na+ concentration near an anionic tetramethylammonium dodecyl sulfate (TMADS) micelle surface was determined with new fluorescent photoinduced electron transfer (PET) sensors. Electrostatic interactions with the negatively charged sulfonate groups of the surfactant induce an increase in the Na+ concentration compared with bulk water.
Abstract
The Na+ concentration near membranes controls our nerve signals aside from several other crucial bioprocesses. Fluorescent photoinduced electron transfer (PET) sensor molecules target Na+ ions in nanospaces near micellar membranes with excellent selectivity against H+. The Na+ concentration near anionic micelles was found to be higher than that in bulk water by factors of up to 160. Sensor molecules that are not held tightly to the micelle surface only detected a Na+ amplification factor of 8. These results were strengthened by the employment of control compounds whose PET processes are permanently “on” or “off”.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201509096_sm_miscellaneous_information.pdf208.1 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. S. Bretscher, Sci. Am. 1985, 253(4), 86–90;
- 1b Molecular Biology of the Cell, 6th ed. ), Garland Science, New York, 2015, pp. 597–640.
- 2F. M. Harold, The Vital Force-A Study of Bioenergetics, Freeman, New York, 1986, pp. 318–332.
- 3
- 3aJ. C. Skou, Biochim. Biophys. Acta 1957, 23, 394–401;
- 3bJ. H. Kaplan, Annu. Rev. Biochem. 2002, 71, 511–535.
- 4K. A. Williams, Nature 2000, 403, 112–115.
- 5J. Payandeh, T. Scheuer, N. Zheng, W. A. Catterall, Nature 2011, 475, 353–358.
- 6J. H. Fendler, Membrane Mimetic Chemistry, Wiley, New York, 1982.
- 7A. P. de Silva, K. R. A. S. Sandanayake, J. Chem. Soc. Chem. Commun. 1989, 1183–1185.
- 8
- 8aM. S. Fernández, P. Fromherz, J. Phys. Chem. 1977, 81, 1755–1761;
- 8bR. A. Bissell, A. J. Bryan, A. P. de Silva, C. P. McCoy, J. Chem. Soc. Chem. Commun. 1994, 405–407;
- 8cS. Uchiyama, K. Iwai, A. P. de Silva, Angew. Chem. Int. Ed. 2008, 47, 4667–4669; Angew. Chem. 2008, 120, 4745–4747.
- 9
- 9aS. Bhattacharya, A. Gulyani, Chem. Commun. 2003, 1158–1159;
- 9bK. Niikura, E. V. Anslyn, J. Org. Chem. 2003, 68, 10156–10157;
- 9cY. Nakahara, T. Kida, Y. Nakatsuji, M. Akashi, Org. Biomol. Chem. 2005, 3, 1787–1794;
- 9dJ. Wang, X. Qian, J. Qian, Y. Xu, Chem. Eur. J. 2007, 13, 7543–7552;
- 9eH. Tian, J. Qian, H. Bai, Q. Sun, L. Zhang, W. Zhang, Anal. Chim. Acta 2013, 768, 136–142;
- 9fN. Kumari, N. Dey, S. Jha, S. Bhattacharya, ACS Appl. Mater. Interfaces 2013, 5, 2438–2445.
- 10
- 10aS. Uchiyama, G. D. McClean, K. Iwai, A. P. de Silva, J. Am. Chem. Soc. 2005, 127, 8920–8921;
- 10bY. Diaz-Fernandez, F. Foti, C. Mangano, P. Pallavicini, S. Patroni, A. Perez-Gramatges, S. Rodriguez-Calvo, Chem. Eur. J. 2006, 12, 921–930;
- 10cA. P. de Silva, C. M. Dobbin, T. P. Vance, B. Wannalerse, Chem. Commun. 2009, 1386–1389;
- 10dP. Pallavicini, Y. A. Diaz-Fernandez, L. Pasotti, Coord. Chem. Rev. 2009, 253, 2226–2240.
- 11
- 11aA. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher, T. E. Rice, Chem. Rev. 1997, 97, 1515–1566;
- 11bL. Prodi, New J. Chem. 2005, 29, 20–31;
- 11cJ. F. Callan, A. P. de Silva, D. C. Magri, Tetrahedron 2005, 61, 8551–8588;
- 11dJ. Chan, S. C. Dodani, C. J. Chang, Nat. Chem. 2012, 4, 973–984;
- 11eY. Yang, Q. Zhao, W. Feng, F. Li, Chem. Rev. 2013, 113, 192–270.
- 12
- 12aA. P. de Silva, T. S. Moody, G. D. Wright, Analyst 2009, 134, 2385–2393;
- 12bA. P. de Silva, J. Phys. Chem. Lett. 2011, 2, 2865–2871;
- 12cB. Daly, J. Ling, A. P. de Silva, Chem. Soc. Rev. 2015, 44, 4203–4211.
- 13R. M. Izatt, R. E. Terry, D. P. Nelson, Y. Chan, D. J. Eatough, J. S. Bradshaw, L. D. Hansen, J. J. Christensen, J. Am. Chem. Soc. 1976, 98, 7626–7630.
- 14C. D. Tran, T. A. Van Fleet, Anal. Chem. 1988, 60, 2478–2482.
- 15A. P. de Silva, K. R. A. S. Sandanayake, Tetrahedron Lett. 1991, 32, 421–424.
- 16
- 16aT. Oe, M. Morita, T. Toyo’oka, Anal. Sci. 1999, 15, 1021–1023;
- 16bC. Gota, S. Uchiyama, T. Yoshihara, S. Tobita, T. Ohwada, J. Phys. Chem. B 2008, 112, 2829–2836.
- 17Double control experiments of this kind are rare. In the related but different field of fluorescent pH sensors, reference compounds displaying pH-independent high PET rates and pH-independent low PET rates are known to bracket the pH-dependent behavior of the sensor itself; see: A. P. de Silva, S. A. de Silva, A. S. Dissanayake, K. R. A. S. Sandanayake, J. Chem. Soc. Chem. Commun. 1989, 1054–1056.
- 18Y. Yue, J. Wang, M. Dai, Langmuir 2000, 16, 6114–6117.
- 19For a detailed description of Δlog β
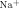 , see the Supporting Information.
, see the Supporting Information.
- 20S. Basili, T. D. Giacco, F. Elisei, R. Germani, Org. Biomol. Chem. 2014, 12, 6677–6683.
- 21K. Sumaru, H. Matsuoka, H. Yamaoka, G. D. Wignall, Phys. Rev. E 1996, 53, 1744–1752.
- 22P. Mukerjee, K. J. Mysels, P. Kapauan, J. Phys. Chem. 1967, 71, 4166–4175.
- 23R. von Wandruszka, Crit. Rev. Anal. Chem. 1992, 23, 187–215.