Incorporation of a Non-Natural Arginine Analogue into a Cyclic Peptide Leads to Formation of Positively Charged Nanofibers Capable of Gene Transfection
Mao Li
Institute for Organic Chemistry, University of Duisburg-Essen, 45117 Essen (Germany)
Search for more papers by this authorMartin Ehlers
Institute for Organic Chemistry, University of Duisburg-Essen, 45117 Essen (Germany)
Search for more papers by this authorStefanie Schlesiger
Institute for Biology, University of Duisburg-Essen, 45117 Essen (Germany)
Search for more papers by this authorElio Zellermann
Institute for Organic Chemistry, University of Duisburg-Essen, 45117 Essen (Germany)
Search for more papers by this authorProf. Shirley K. Knauer
Institute for Biology, University of Duisburg-Essen, 45117 Essen (Germany)
Search for more papers by this authorCorresponding Author
Prof. Carsten Schmuck
Institute for Organic Chemistry, University of Duisburg-Essen, 45117 Essen (Germany)
Institute for Organic Chemistry, University of Duisburg-Essen, 45117 Essen (Germany)Search for more papers by this authorMao Li
Institute for Organic Chemistry, University of Duisburg-Essen, 45117 Essen (Germany)
Search for more papers by this authorMartin Ehlers
Institute for Organic Chemistry, University of Duisburg-Essen, 45117 Essen (Germany)
Search for more papers by this authorStefanie Schlesiger
Institute for Biology, University of Duisburg-Essen, 45117 Essen (Germany)
Search for more papers by this authorElio Zellermann
Institute for Organic Chemistry, University of Duisburg-Essen, 45117 Essen (Germany)
Search for more papers by this authorProf. Shirley K. Knauer
Institute for Biology, University of Duisburg-Essen, 45117 Essen (Germany)
Search for more papers by this authorCorresponding Author
Prof. Carsten Schmuck
Institute for Organic Chemistry, University of Duisburg-Essen, 45117 Essen (Germany)
Institute for Organic Chemistry, University of Duisburg-Essen, 45117 Essen (Germany)Search for more papers by this authorGraphical Abstract
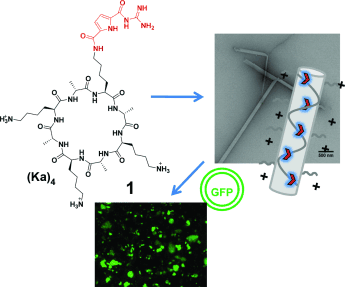
Delivering the goods: After functionalization of the cyclic peptide (Ka)4 with a single guanidiniocarbonyl pyrrole (GCP) moiety, cationic nanofibers of micrometer length are formed. These aggregates are efficient gene transfection vectors. DNA binds to their cationic surface and is efficiently delivered into cells. GFP=green fluorescent protein.
Abstract
Functionalization of the tetracationic cyclic peptide (Ka)4 with a single guanidiniocarbonyl pyrrole (GCP) moiety, a weakly basic but highly efficient arginine analogue, completely alters the self-assembly properties of the peptide. In contrast to the nonfunctionalized peptide 2, which does not self-assemble, GCP-containing peptide 1 forms cationic nanofibers of micrometer length. These aggregates are efficient gene transfection vectors. DNA binds to their cationic surface and is efficiently delivered into cells.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201508714_sm_miscellaneous_information.pdf7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. A. Mintzer, E. E. Simanek, Chem. Rev. 2009, 109, 259–302;
- 1bH. Margus, K. Padari, M. Pooga, Mol. Ther. 2012, 20, 525–533.
- 2S. Bhattacharya, A. Bajaj, Chem. Commun. 2009, 4632–4656.
- 3
- 3aS. Futaki, W. Ohashi, T. Suzuki, M. Niwa, S. Tanaka, K. Ueda, H. Harashima, Y. Sugiura, Bioconjugate Chem. 2001, 12, 1005–1011;
- 3bW. J. Kim, L. V. Christensen, S. Jo, J. W. Yockman, J. H. Jeong, Y. H. Kim, S. W. Kim, Mol. Ther. 2006, 14, 343–350;
- 3cC. Gehin, J. Montenegro, E. K. Bang, A. Cajaraville, S. Takayama, H. Hirose, S. Futaki, S. Matile, H. Riezman, J. Am. Chem. Soc. 2013, 135, 9295–9298.
- 4M. Yolamanova, C. Meier, A. K. Shaytan, V. Vas, C. W. Bertoncini, F. Arnold, O. Zirafi, S. M. Usmani, J. A. Muller, D. Sauter, C. Goffinet, D. Palesch, P. Walther, N. R. Roan, H. Geiger, O. Lunov, T. Simmet, J. Bohne, H. Schrezenmeier, K. Schwarz, L. Standker, W. G. Forssmann, X. Salvatella, P. G. Khalatur, A. R. Khokhlov, T. P. J. Knowles, T. Weil, F. Kirchhoff, J. Munch, Nat. Nanotechnol. 2013, 8, 130–136.
- 5X. H. Yan, Q. He, K. W. Wang, L. Duan, Y. Cui, J. B. Li, Angew. Chem. Int. Ed. 2007, 46, 2431–2434; Angew. Chem. 2007, 119, 2483–2486.
- 6
- 6aS. G. Zhang, Acc. Chem. Res. 2012, 45, 2142–2150;
- 6bH. G. Cui, M. J. Webber, S. I. Stupp, Biopolymers 2010, 94, 1–18;
- 6cH. Hosseinkhani, P. D. Hong, D. S. Yu, Chem. Rev. 2013, 113, 4837–4861;
- 6dJ. B. Matson, S. I. Stupp, Chem. Commun. 2012, 48, 26–33;
- 6eS. Kim, J. H. Kim, J. S. Lee, C. B. Park, Small 2015, 11, 3623–3640;
- 6fH. Jang, F. T. Arce, S. Ramachandran, B. L. Kagan, R. Lal, R. Nussinov, Chem. Soc. Rev. 2014, 43, 6750–6764.
- 7X. B. Zhao, F. Pan, H. Xu, M. Yaseen, H. H. Shan, C. A. E. Hauser, S. G. Zhang, J. R. Lu, Chem. Soc. Rev. 2010, 39, 3480–3498.
- 8I. W. Hamley, Angew. Chem. Int. Ed. 2014, 53, 6866–6881; Angew. Chem. 2014, 126, 6984–7000.
- 9
- 9aM. R. Ghadiri, J. R. Granja, R. A. Milligan, D. E. Mcree, N. Khazanovich, Nature 1993, 366, 324–327;
- 9bR. J. Brea, C. Reiriz, J. R. Granja, Chem. Soc. Rev. 2010, 39, 1448–1456.
- 10
- 10aS. Fernandez-Lopez, H. S. Kim, E. C. Choi, M. Delgado, J. R. Granja, A. Khasanov, K. Kraehenbuehl, G. Long, D. A. Weinberger, K. M. Wilcoxen, M. R. Ghadiri, Nature 2001, 412, 452–455;
- 10bR. Chapman, M. Danial, M. L. Koh, K. A. Jolliffe, S. Perrier, Chem. Soc. Rev. 2012, 41, 6023–6041;
- 10cJ. Sánchez-Ouesada, M. P. Isler, M. R. Ghadiri, J. Am. Chem. Soc. 2002, 124, 10004–10005;
- 10dM. S. Vollmer, T. D. Clark, C. Steinem, M. R. Ghadiri, Angew. Chem. Int. Ed. 1999, 38, 1598–1601;
10.1002/(SICI)1521-3773(19990601)38:11<1598::AID-ANIE1598>3.0.CO;2-J CAS PubMed Web of Science® Google ScholarAngew. Chem. 1999, 111, 1703–1706;10.1002/(SICI)1521-3757(19990601)111:11<1703::AID-ANGE1703>3.0.CO;2-4 Web of Science® Google Scholar
- 10eM. Danial, S. Perrier, K. A. Jolliffe, Org. Biomol. Chem. 2015, 13, 2464–2473;
- 10fG. Kulsi, A. Ghorai, B. Achari, P. Chattopadhyay, RSC Adv. 2015, 5, 64675–64681;
- 10gG. Aragay, B. Ventura, A. Guerra, I. Pintre, C. Chiorboli, R. Garcia-Fandino, L. Flamigni, J. R. Granja, P. Ballester, Chem. Eur. J. 2014, 20, 3427–3438;
- 10hB. M. Blunden, R. Chapman, M. Danial, H. X. Lu, K. A. Jolliffe, S. Perrier, M. H. Stenzel, Chem. Eur. J. 2014, 20, 12745–12749;
- 10iM. Panciera, M. Amorin, J. R. Granja, Chem. Eur. J. 2014, 20, 10260–10265;
- 10jM. Amorín, A. Pérez, J. Barberá, H. L. Ozores, J. L. Serrano, J. R. Granja, T. Sierra, Chem. Commun. 2014, 50, 688–690.
- 11
- 11aR. Hourani, C. Zhang, R. van der Weegen, L. Ruiz, C. Y. Li, S. Keten, B. A. Helms, T. Xu, J. Am. Chem. Soc. 2011, 133, 15296–15299;
- 11bC. Tarabout, S. Roux, F. Gobeaux, N. Fay, E. Pouget, C. Meriadec, M. Ligeti, D. Thomas, M. Ijsselstijn, F. Besselievre, D. A. Buisson, J. M. Verbavatz, M. Petitjean, C. Valery, L. Perrin, B. Rousseau, F. Artzner, M. Paternostre, J. C. Cintrat, Proc. Natl. Acad. Sci. USA 2011, 108, 7679–7684;
- 11cM. Danial, C. M. N. Tran, K. A. Jolliffe, S. Perrier, J. Am. Chem. Soc. 2014, 136, 8018–8026;
- 11dD. J. Rubin, S. Amini, F. Zhou, H. B. Su, A. Miserez, N. S. Joshi, ACS Nano 2015, 9, 3360–3368.
- 12
- 12aC. Schmuck, W. Wienand, J. Am. Chem. Soc. 2003, 125, 452–459;
- 12bS. Schlund, C. Schmuck, B. Engels, J. Am. Chem. Soc. 2005, 127, 11115–11124.
- 13M. Li, S. Schlesiger, S. K. Knauer, C. Schmuck, Angew. Chem. Int. Ed. 2015, 54, 2941–2944; Angew. Chem. 2015, 127, 2984–2987.
- 14J. Sanchez-Quesada, M. R. Ghadiri, H. Bayley, O. Braha, J. Am. Chem. Soc. 2000, 122, 11757–11766.