Palladium-Catalyzed Heteroarylation and Concomitant ortho-Alkylation of Aryl Iodides
Dr. Chuanhu Lei
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorXiaojia Jin
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jianrong (Steve) Zhou
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)Search for more papers by this authorDr. Chuanhu Lei
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorXiaojia Jin
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jianrong (Steve) Zhou
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)Search for more papers by this authorGraphical Abstract
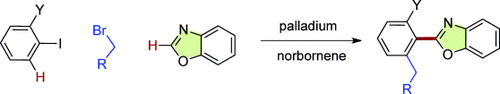
Three-in-one: The heteroarylation of aryl iodides and ortho-alkylation of the aryl rings occur in a single operation. The palladium and norbornene cocatalyst system effectively promoted the cleavage of two CH bonds and the formation of two new CC bonds, including a very hindered aryl–heteroaryl bond.
Abstract
Three-component couplings were achieved from common aryl halides, alkyl halides, and heteroarenes under palladium and norbornene co-catalysis. The reaction forges hindered aryl–heteroaryl bonds and introduces ortho-alkyl groups to aryl rings. Various heterocycles such as oxazoles, thiazoles and thiophenes underwent efficient coupling. The heteroarenes were deprotonated in situ by bases without the assistance of palladium catalysts.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201507128_sm_miscellaneous_information.pdf8.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Examples of aryloxazoles in medicines:
- 1aD. Kumar, M. R. Jacob, M. B. Reynolds, S. M. Kerwin, Bioorg. Med. Chem. 2002, 10, 3997;
- 1bH. Razavi, S. K. Palaninathan, E. T. Powers, R. L. Wiseman, H. E. Purkey, N. N. Mohamedmohaideen, S. Deechongkit, K. P. Chiang, M. T. A. Dendle, J. C. Sacchettini, J. W. Kelly, Angew. Chem. Int. Ed. 2003, 42, 2758; Angew. Chem. 2003, 115, 2864;
- 1cM. S. Malamas, E. S. Manas, R. E. McDevitt, I. Gunawan, Z. B. Xu, M. D. Collini, C. P. Miller, T. Dinh, R. A. Henderson, J. C. Keith, Jr., H. A. Harris, J. Med. Chem. 2004, 47, 5021;
- 1d“Erythropoietin production promoters containing 2-arylbenzoxazoles, and preventive or therapeutic agents for anemia”: T. Nishiyama, JP2011168515A, 2011.
- 2Examples and reviews:
- 2aO. Navarro, R. A. Kelly, S. P. Nolan, J. Am. Chem. Soc. 2003, 125, 16194;
- 2bG. Altenhoff, R. Goddard, C. W. Lehmann, F. Glorius, J. Am. Chem. Soc. 2004, 126, 15195;
- 2cJ.-P. Corbet, G. Mignani, Chem. Rev. 2006, 106, 2651;
- 2dR. Martin, S. L. Buchwald, Acc. Chem. Res. 2008, 41, 1461;
- 2eM. G. Organ, S. Çalimsiz, M. Sayah, K. H. Hoi, A. J. Lough, Angew. Chem. Int. Ed. 2009, 48, 2383; Angew. Chem. 2009, 121, 2419;
- 2fQ. Zhao, C. Li, C. H. Senanayake, W. Tang, Chem. Eur. J. 2013, 19, 2261.
- 3Examples and reviews:
- 3aD. Alberico, M. E. Scott, M. Lautens, Chem. Rev. 2007, 107, 174;
- 3bJ.-C. Hierso, R. Smaliy, R. Amardeil, P. Meunier, Chem. Soc. Rev. 2007, 36, 1754;
- 3cX. Chen, K. M. Engle, D.-H. Wang, J.-Q. Yu, Angew. Chem. Int. Ed. 2009, 48, 5094; Angew. Chem. 2009, 121, 5196;
- 3dO. Daugulis, H.-Q. Do, D. Shabashov, Acc. Chem. Res. 2009, 42, 1074;
- 3eG. P. McGlacken, L. M. Bateman, Chem. Soc. Rev. 2009, 38, 2447;
- 3fD. A. Colby, R. G. Bergman, J. A. Ellman, Chem. Rev. 2010, 110, 624;
- 3gT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147;
- 3hD. Roy, S. Mom, M. Beaupérin, H. Doucet, J.-C. Hierso, Angew. Chem. Int. Ed. 2010, 49, 6650; Angew. Chem. 2010, 122, 6800;
- 3iL. Ackermann, Chem. Rev. 2011, 111, 1315;
- 3jD. Roy, S. Mom, D. Lucas, H. Cattey, J.-C. Hierso, H. Doucet, Chem. Eur. J. 2011, 17, 6453;
- 3kB.-J. Li, Z.-J. Shi, Chem. Soc. Rev. 2012, 41, 5588;
- 3lJ. Yamaguchi, A. D. Yamaguchi, K. Itami, Angew. Chem. Int. Ed. 2012, 51, 8960; Angew. Chem. 2012, 124, 9092;
- 3mJ. Wencel-Delord, F. Glorius, Nat. Chem. 2013, 5, 369;
- 3nM. Miura, T. Satoh, K. Hirano, Bull. Chem. Soc. Jpn. 2014, 87, 751;
- 3oR. Rossi, F. Bellina, M. Lessi, C. Manzini, Adv. Synth. Catal. 2014, 356, 17.
- 4Reviews:
- 4aS.-L. You, J.-B. Xia, Top. Curr. Chem. 2010, 292, 165;
- 4bS. H. Cho, J. Y. Kim, J. Kwak, S. Chang, Chem. Soc. Rev. 2011, 40, 5068;
- 4cC. S. Yeung, V. M. Dong, Chem. Rev. 2011, 111, 1215;
- 4dN. Kuhl, M. N. Hopkinson, J. Wencel-Delord, F. Glorius, Angew. Chem. Int. Ed. 2012, 51, 10236; Angew. Chem. 2012, 124, 10382.
- 5Examples:
- 5aB.-J. Li, S.-L. Tian, Z. Fang, Z.-J. Shi, Angew. Chem. Int. Ed. 2008, 47, 1115; Angew. Chem. 2008, 120, 1131;
- 5bM. Wasa, B. T. Worrell, J.-Q. Yu, Angew. Chem. Int. Ed. 2010, 49, 1275; Angew. Chem. 2010, 122, 1297;
- 5cX. Zhao, C. S. Yeung, V. M. Dong, J. Am. Chem. Soc. 2010, 132, 5837;
- 5dJ. Wencel-Delord, C. Nimphius, F. W. Patureau, F. Glorius, Angew. Chem. Int. Ed. 2012, 51, 2247; Angew. Chem. 2012, 124, 2290.
- 6Examples:
- 6aD. García-Cuadrado, A. A. C. Braga, F. Maseras, A. M. Echavarren, J. Am. Chem. Soc. 2006, 128, 1066;
- 6bD. R. Stuart, K. Fagnou, Science 2007, 316, 1172;
- 6cD. R. Stuart, E. Villemure, K. Fagnou, J. Am. Chem. Soc. 2007, 129, 12072;
- 6dN. Guimond, S. I. Gorelsky, K. Fagnou, J. Am. Chem. Soc. 2011, 133, 6449;
- 6eG. Wu, J. Zhou, M. Zhang, P. Hu, W. Su, Chem. Commun. 2012, 48, 8964.
- 7Examples and reviews:
- 7aL. Ackermann, R. Vicente, A. R. Kapdi, Angew. Chem. Int. Ed. 2009, 48, 9792; Angew. Chem. 2009, 121, 9976;
- 7bP. Xi, F. Yang, S. Qin, D. Zhao, J. Lan, G. Gao, C. Hu, J. You, J. Am. Chem. Soc. 2010, 132, 1822;
- 7cD. Mandal, A. D. Yamaguchi, J. Yamaguchi, K. Itami, J. Am. Chem. Soc. 2011, 133, 19660;
- 7dD. Zhao, J. You, C. Hu, Chem. Eur. J. 2011, 17, 5466;
- 7eK. Hirano, M. Miura, Chem. Commun. 2012, 48, 10704;
- 7fK. Hirano, M. Miura, Top. Catal. 2014, 57, 878.
- 8Examples:
- 8aJ. Kwak, M. Kim, S. Chang, J. Am. Chem. Soc. 2011, 133, 3780;
- 8bT. W. Lyons, K. L. Hull, M. S. Sanford, J. Am. Chem. Soc. 2011, 133, 4455;
- 8cX. Wang, D. Leow, J.-Q. Yu, J. Am. Chem. Soc. 2011, 133, 13864;
- 8dM. Ye, G.-L. Gao, A. J. F. Edmunds, P. A. Worthington, J. A. Morris, J.-Q. Yu, J. Am. Chem. Soc. 2011, 133, 19090.
- 9Examples:
- 9aC. Bressy, D. Alberico, M. Lautens, J. Am. Chem. Soc. 2005, 127, 13148;
- 9bC. Blaszykowski, E. Aktoudianakis, C. Bressy, D. Alberico, M. Lautens, Org. Lett. 2006, 8, 2043;
- 9cA. Martins, D. Alberico, M. Lautens, Org. Lett. 2006, 8, 4827;
- 9dK. Mitsudo, P. Thansandote, T. Wilhelm, B. Mariampillai, M. Lautens, Org. Lett. 2006, 8, 3939;
- 9eC. Blaszykowski, E. Aktoudianakis, D. Alberico, C. Bressy, D. G. Hulcoop, F. Jafarpour, A. Joushaghani, B. Laleu, M. Lautens, J. Org. Chem. 2008, 73, 1888.
- 10Examples:
- 10aS. J. Tremont, R. Hayat ur, J. Am. Chem. Soc. 1984, 106, 5759;
- 10bG. Bocelli, M. Catellani, S. Ghelli, J. Organomet. Chem. 1993, 458, C 12.
- 11Reviews:
- 11aM. Catellani, Synlett 2003, 298;
- 11bM. Catellani, E. Motti, N. Della Ca’, R. Ferraccioli, Eur. J. Org. Chem. 2007, 4153;
- 11cM. Catellani, E. Motti, N. Della Ca’, Acc. Chem. Res. 2008, 41, 1512;
- 11dG. P. Chiusoli, M. Catellani, M. Costa, E. Motti, N. Della Ca’, G. Maestri, Coord. Chem. Rev. 2010, 254, 456;
- 11eA. Martins, B. Mariampillai, M. Lautens, Top. Curr. Chem. 2010, 292, 1;
- 11fP. Sehnal, R. J. K. Taylor, I. J. S. Fairlamb, Chem. Rev. 2010, 110, 824;
- 11gR. Ferraccioli, Synthesis 2013, 581.
- 12Examples:
- 12aM. Catellani, F. Frignani, A. Rangoni, Angew. Chem. Int. Ed. Engl. 1997, 36, 119; Angew. Chem. 1997, 109, 142;
- 12bM. Catellani, E. Motti, M. Minari, Chem. Commun. 2000, 157;
- 12cN. Della Ca’, G. Maestri, M. Catellani, Chem. Eur. J. 2009, 15, 7850.
- 13Examples:
- 13aA. Rudolph, N. Rackelmann, M. Lautens, Angew. Chem. Int. Ed. 2007, 46, 1485; Angew. Chem. 2007, 119, 1507;
- 13bK. M. Gericke, D. I. Chai, N. Bieler, M. Lautens, Angew. Chem. Int. Ed. 2009, 48, 1447; Angew. Chem. 2009, 121, 1475;
- 13cM. Blanchot, D. A. Candito, F. Larnaud, M. Lautens, Org. Lett. 2011, 13, 1486;
- 13dH. Weinstabl, M. Suhartono, Z. Qureshi, M. Lautens, Angew. Chem. Int. Ed. 2013, 52, 5305; Angew. Chem. 2013, 125, 5413.
- 14Examples:
- 14aX. Sui, R. Zhu, G. Li, X. Ma, Z. Gu, J. Am. Chem. Soc. 2013, 135, 9318;
- 14bH. Zhang, P. Chen, G. Liu, Angew. Chem. Int. Ed. 2014, 53, 10174; Angew. Chem. 2014, 126, 10338.
- 15Examples of arylation of oxazoles:
- 15aH.-Q. Do, O. Daugulis, J. Am. Chem. Soc. 2007, 129, 12404;
- 15bL. Ackermann, A. Althammer, S. Fenner, Angew. Chem. Int. Ed. 2009, 48, 201; Angew. Chem. 2009, 121, 207;
- 15cJ. Canivet, J. Yamaguchi, I. Ban, K. Itami, Org. Lett. 2009, 11, 1733;
- 15dH. Hachiya, K. Hirano, T. Satoh, M. Miura, Org. Lett. 2009, 11, 1737;
- 15eB. M. Rosen, K. W. Quasdorf, D. A. Wilson, N. Zhang, A.-M. Resmerita, N. K. Garg, V. Percec, Chem. Rev. 2011, 111, 1346.
- 16
- 16aC. Amatore, A. Jutand, Coord. Chem. Rev. 1998, 178;
- 16bS. Pivsa-Art, T. Satoh, Y. Kawamura, M. Miura, M. Nomura, Bull. Chem. Soc. Jpn. 1998, 71, 467;
- 16cN. G. Andersen, B. A. Keay, Chem. Rev. 2001, 101, 997.
- 17K. Shen, Y. Fu, J.-N. Li, L. Liu, Q.-X. Guo, Tetrahedron 2007, 63, 1568.
- 18Synthesis of norbornene-derived palladacycles:
- 18aD. I. Chai, P. Thansandote, M. Lautens, Chem. Eur. J. 2011, 17, 8175;
- 18bX. Wu, J. Zhou, Chem. Commun. 2013, 49, 11035.
- 19X. Wu, C. Lei, G. Yue, J. Zhou, Angew. Chem. Int. Ed. 2015, 54, 9601; Angew. Chem. 2015, 127, 9737.
- 20R. S. Sánchez, F. A. Zhuravlev, J. Am. Chem. Soc. 2007, 129, 5824.
- 21T. Yamamoto, K. Muto, M. Komiyama, J. Canivet, J. Yamaguchi, K. Itami, Chem. Eur. J. 2011, 17, 10113.