Methane as a Selectivity Booster in the Arc-Discharge Synthesis of Endohedral Fullerenes: Selective Synthesis of the Single-Molecule Magnet Dy2TiC@C80 and Its Congener Dy2TiC2@C80
Graphical Abstract
Abstract
The use of methane as a reactive gas dramatically increases the selectivity of the arc-discharge synthesis of M-Ti-carbide clusterfullerenes (M=Y, Nd, Gd, Dy, Er, Lu). Optimization of the process parameters allows the synthesis of Dy2TiC@C80-I and its facile isolation in a single chromatographic step. A new type of cluster with an endohedral acetylide unit, M2TiC2@C80, is discovered along with the second isomer of M2TiC@C80. Dy2TiC@C80-(I,II) and Dy2TiC2@C80-I are shown to be single-molecule magnets (SMM), but the presence of the second carbon atom in the cluster Dy2TiC2@C80 leads to substantially poorer SMM properties.
The field of endohedral metallofullerene (EMF) research was revolutionized in 1999, when it was discovered that the presence of small amounts of nitrogen gas in the arc-discharge generator afforded Sc3N@C80, a new type of EMF with a trimetalnitride cluster inside the carbon cage.1 The use of NH3 as a reactive gas instead of molecular nitrogen resulted in much higher selectivity in the synthesis of nitride clusterfullerenes as the yield of empty fullerenes in such conditions decreased dramatically.2 Discovery of nitride clusterfullerenes triggered exhaustive studies of other clusterfullerenes, resulting in a variety of EMF families with endohedral S,3 O,4 C2,5 CH,6 CN,7 and other nonmetal units.8
One of the advantages of the trimetallic cluster in nitride clusterfullerenes is the possibility of combining two or even three different metals within one EMF molecule. Mixed-metal nitride clusterfullerenes may exhibit new properties not present in homometallic nitride clusterfullerenes. Examples include unusual redox behavior,9 stabilization of unconventional carbon cages,10 and strong variation of chemical reactivity11 and magnetization behavior12 depending on the number of lanthanide ions in the cluster. However, a disadvantage of the mixed-metal EMFs is the increased complexity of their chromatographic separation.
Whereas nitride clusterfullerenes are usually formed with Group III metals, such as Sc, Y, and trivalent lanthanides,13 Yang et al. demonstrated that a single Ti ion can be introduced into the mixed-metal nitride cluster together with Sc or Y.14 Due to the trivalent Ti, M2TiN@C80 clusterfullerenes have unusual electronic and chemical properties.15 Recently, in an attempt to obtain Ti-based nitride clusterfullerenes with Lu using NH3 as a reactive gas or melamine as a solid organic nitrogen source, we have discovered a new type of clusterfullerene, Lu2TiC@C80, which has an endohedral μ3-carbide ion and a TiC double bond.16 The molecule is an isostructural analogue of Lu2ScN@C80, in which the Sc–N fragment is replaced by the isoelectronic TiC fragment. Unfortunately, in the Lu/Ti/NH3 and Lu/Ti/melamine syntheses, Lu2TiC@C80 is only a minor by-product; the products are predominantly Lu3N@C2n nitride clusterfullerenes, which precludes further exploration of this new type of clusterfullerenes. Herein we demonstrate that 1) M2TiC@C80 clusterfullerenes can be synthesized with high selectivity for many lanthanides using methane as a reactive gas; 2) this synthetic route also affords appreciable amounts of another cage isomer of M2TiC@C80 as well as a new type of clusterfullerenes, M2TiC2@C80; 3) Dy2TiC@C80 exhibits single-molecule magnet (SMM) behavior, whereas the SMM properties of Dy2TiC2@C80 are are less pronounced owing to the presence of the second carbon atom in the central unit.
EMFs were obtained by arc-discharge synthesis under He atmosphere with a small amount of CH4 gas using graphite rods packed with a mixture of Ti, lanthanide (Y, Ce, Nd, Gd, Dy, Er, or Lu), and a graphite powder. The role of CH4 in this type of synthesis is similar to that of NH32 in the synthesis of nitride clusterfullerenes: the reactive gas increases selectivity of the process by suppressing the formation of empty fullerenes and making the EMFs with desired central atom(s) the main products. Figure 1 shows that under optimized conditions, carbide clusterfullerenes are the most abundant EMF products for Lu, Dy, Er, Y, and Gd. M2TiC@C80-I (Roman number denotes the isomer) is the major or the sole component of the fraction eluting at 36 min. Thus, pure M2TiC@C80-I (Lu, Dy, Gd) was obtained from the EMF extract in a single HPLC separation step. For other metals, the main fraction also included a small amount of M2C82, which could be then easily removed at the second HPLC step (see the Supporting Information (SI), Figures S2 and S4).
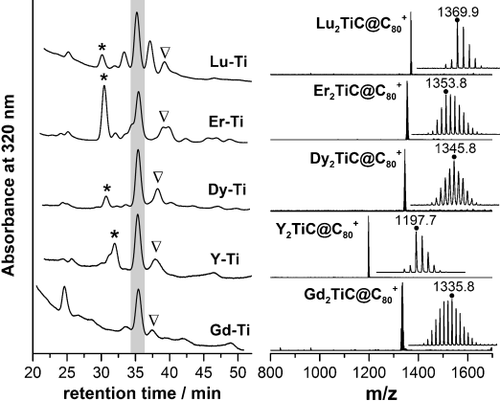
Left: HPLC chromatograms of raw extracts obtained in metal/Ti/CH4 arc-discharge syntheses (Buckyprep column, toluene as eluent); asterisks mark the fractions with M3N@C80, shaded rectangle highlights the fractions with M2TiC@C80-I, whereas triangles denote the fractions with M2TiC@C80-II/M2TiC2@C80-I. Right: positive-ion MALDI mass spectra of isolated M2TiC@C80-I; insets show isotopic distributions and mass numbers of the most intense peaks (marked with the dot).
The ionic radius of the lanthanide ion (R3+) plays a crucial role in the absolute yield of EMFs. Lu (R3+=0.86 Å), Er (0.90 Å), and Dy (0.91 Å) afford similar amounts of M2TiC@C80-I per synthesis, the yields of Gd2TiC@C80-I (0.94 Å) and Nd2TiC@C80-I (0.98 Å) are roughly 6 and 20 times lower than that of Dy2TiC@C80-I, respectively, whereas Ce2TiC@C80 (1.01 Å) is not produced at all. Yttrium (R3+=0.90 Å) stands apart as the yield of Y2TiC@C80-I is about 4 times lower than that of Dy2TiC@C80-I, despite the similar ionic radii of Dy3+ and Y3+.
Significant amounts of M2C82 and M2C84 (presumably dimetallofullerenes M2@C82 and carbides M2C2@C82) are formed for Lu and Er. Besides, the presence of traces of nitrogen in the generator leads to the formation of nitride clusterfullerene M3N@C80. However, the yield of these EMFs decreases with the ionic radius faster than the yield of M2TiC@C80-I, and Dy2TiC@C80-I can be produced with a high degree of selectivity and relatively high absolute yield. Equally high selectivity is also achieved for Y and Gd (Figure 1), but their overall yield is lower.
The molecular structure of Lu2TiC@C80 determined by single-crystal X-ray diffraction and 13C NMR spectroscopy was reported earlier.16 The Lu2TiC cluster rotates inside the C80-Ih(7) fullerene resulting in a simple two-line 13C NMR spectrum of the cage carbons. The use of 13C powder for the synthesis of Lu2TiC@C80-I in this work allowed detection of the 13C resonance of the central carbon atom at 340.98 ppm (Figure 2). This value is much more positive than the chemical shift of endohedral carbon atoms reported before: 220–260 ppm in M2C2@C2n clusterfullerenes,8c 292.4 ppm in YCN@C82,17 and 328.3 ppm in the Sc3C2@C80− anion.18 Note that large downfield shifts for metal-bonded carbon atoms are not unusual in Ti alkylidenes with a TiC bond.19 Particularly large shifts in the range of 400–600 ppm are reported for α-C atoms in μ3-bridging alkylidynes of titanium in titanocubane frameworks.20

a) 13C NMR spectrum of 13C-enriched Lu2TiC@C80-I, asterisks mark the lines of the solvent and the lock. b) Vis/NIR absorption spectra of M2TiC@C80-I in toluene. c) FTIR spectra of M2TiC@C80-I (gray: M=Lu; black: M=Dy).The inset in (c) compares two spectra in the range of the TiC stretching mode.
The high selectivity makes Dy2TiC@C80-I a suitable synthetic target for further studies of its properties. Comparison of the Vis/NIR and IR absorption spectra prove that the Dy and Lu compounds are isostructural (Figure 2). Both have very similar Vis/NIR spectra with the lowest energy band at 910 nm (Figure 2 b) and almost identical vibrational pattern of the fullerene cage (Figure 2 c). However, the larger ionic radius of Dy3+ pushes the central carbon atom closer to Ti, which results in a higher TiC stretching frequency in Dy2TiC@C80-I (834 cm−1 versus 821 cm−1 in Lu2TiC@C80-I). Analogous variations of the vibrational frequency with an increase of the lanthanide ionic radius was observed for the ScN stretching mode in M2ScN@C80.21 In a similar fashion, isostructurality of other M2TiC@C80-I EMFs can be established via their absorption spectra (Figure S7). Besides, all compounds show similar redox behavior with the reversible Ti-based reduction near −1.0 V (vs. Fe(Cp)2+/0 couple) and cage-based oxidation at +0.6 V (Figure 3, Figure S8, and Table 1). Note that reduction potential depends on the size of the lanthanide, whereas the oxidation process is metal-independent.
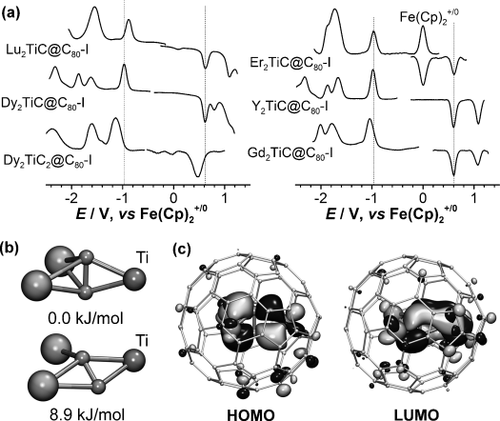
a) Square-wave voltammetry of M2TiC@C80-I (M=Lu, Dy, Er, Y, Gd) and Dy2TiC2@C80-I measured in o-dichlorobenzene/tetrabutylammonium hexafluorophosphate (o-DCB/TBAPF6). b) Two conformations of the Lu2TiC2 cluster in the DFT-optimized molecular structure of Lu2TiC2@C80-I (atoms are shown as spheres with the radius decreasing from Lu to Ti to C). c) HOMO and LUMO of Lu2TiC2@C80-I computed at the PBE/TZVP level.
|
EMF |
Ox-II |
Ox-I |
Red-I |
Red-II |
Red-III |
Red-IV |
|---|---|---|---|---|---|---|
|
Lu2TiC@C80-I |
1.10 |
0.63 |
−0.87 |
−1.53 |
– |
– |
|
Er2TiC@C80-I |
0.62 |
−0.96 |
−1.72 |
– |
– |
|
|
Y2TiC@C80-I |
1.07 |
0.60 |
−0.99 |
−1.67 |
−1.89 |
−2.32 |
|
Gd2TiC@C80-I |
1.07 |
0.60 |
−1.04 |
−1.72 |
−1.91 |
−2.44 |
|
Dy2TiC@C80-I |
0.61 |
−0.97 |
−1.62 |
−1.87 |
−2.33 |
|
|
Dy2TiC2@C80-I |
0.47 |
−1.14 |
−1.58 |
−2.29 |
– |
- [a] All potentials are determined by square-wave voltammetry and are reference to the Fe(Cp)2+/0 redox couple; the values for Lu2TiC@C80-I are from Ref. 16.
Owing to the higher selectivity in the synthesis with CH4 as the reactive gas, other Ln–Ti carbide clusterfullerenes could be detected; this was not possible in the first report on Lu2TiC@C80-I.16 Mass spectrometry studies proved the formation of M2TiC@C2n with larger cages (C82, C84), albeit in rather small amounts making their isolation impractical at this moment (see SI). More importantly, we identified the second isomer of M2TiC@C80 and a new type of M-Ti-carbide cluster with one more carbon atom in the structure, M2TiC2@C80 (the new clusterfullerene also has two isomers, see Figures S1 b and S2 a). M2TiC@C80-II and M2TiC2@C80-I have very similar retention times and elute in one fraction (Figures 1 and 4). Dy and Lu EMFs were separated by recycling HPLC as shown in Figures S1 and S3. Figure 4 compares HPLC traces and mass spectra of isolated Dy2TiC@C80-II and Dy2TiC2@C80-I (each sample still contains a few percent of the other compound). Absorption spectra of M2TiC@C80-II and M2TiC2@C80-I (M=Dy, Lu) shown in Figure 4 b,c lack well-defined features and extend to ca. 1000 nm for the former and ca. 1100 for the latter. The close similarity of the M2TiC and M2ScN clusters suggest that M2TiC@C80-II is likely to have a D5h(6) cage. The 13C NMR spectrum of Lu2TiC2@C80-I (Figure 4 c inset) has two 13C signals at 143.20 and 136.05 ppm (versus 143.46 and 136.60 ppm in Lu2TiC@C80-I). Thus, the cage symmetry of M2TiC2@C80-I can be assigned as Ih(7).
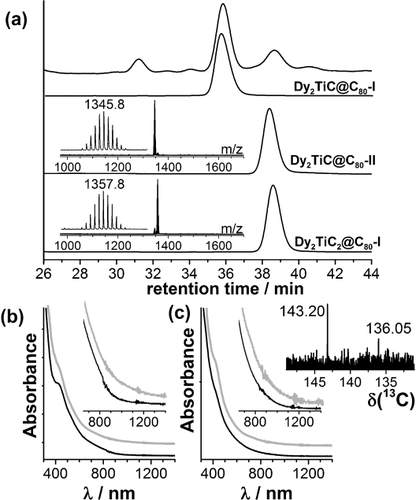
a) HPLC trace of the raw Dy-Ti extract (top) and HPLC curves for individual Dy2TiC@C80-I, Dy2TiC@C80-II, and Dy2TiC2@C80-I; the insets show their mass spectra. b,c) UV/Vis/NIR absorption spectra of (b) M2TiC@C80-II and (c) M2TiC2@C80-I (black: M=Dy, gray: M=Lu); the inset shows the 13C NMR spectrum of Lu2TiC2@C80-I.
DFT calculations show that the M2TiC2 cluster has two conformations with similar energies. In the most stable one, the C2 unit is perpendicular to the M2Ti plane and has μ2-coordination with all metal atoms (Figure 3). In the second one (9 kJ mol−1 higher in energy), the C2 fragment is tilted out of the M2Ti plane, has μ2-coordination with Ti and one lanthanide, and μ1-coordination with another lanthanide. C2 is likely to exhibit fluxional motion at room temperature. The structure and dynamics of the M2TiC2 cluster are similar to those of M3C2 clusters in Sc3C2@C8022 and Lu3C2@C88.23
M2TiC@C80-I has a cluster-localized LUMO with large contribution of Ti and a fullerene-based HOMO,16 and similar spatial distribution of the frontier MOs is predicted for M2TiC@C80-II. In Lu2TiC2@C80-I, both HOMO and LUMO are predominantly localized on the endohedral cluster and have a large contribution of the acetylide fragment. The LUMO of Lu2TiC2@C80 is to a large extent localized on Ti, whereas in the HOMO both Lu and Ti have comparable contributions (Figure 3). Thus, both reduction and oxidation of M2TiC2@C80-I are cluster-based processes. Dy2TiC2@C80-I exhibits two reversible reductions and one oxidation; in comparison to Dy2TiC@C80-I, the redox potentials are shifted cathodically by ca. 0.15 V (Figure 3, Table 1).
Recently we have discovered that mixed-metal nitride clusterfullerenes DyxSc−-xN@C80 (x=1–3) exhibit slow relaxation of the magnetization with a temperature-dependent decay rate, that is, they behave as single-molecule magnets (SMMs).12, 24 Of the three nitride clusterfullerenes, Dy2ScN@C80 is the strongest SMM due to the ferromagnetic exchange and dipolar interaction of the two Dy3+ ions. Unfortunately, selective synthesis of mixed-metal nitride clusterfullerenes is not possible. The arc-discharge synthesis in the Dy–Sc system always produces a mixture of DyxSc3−xN@C2n (x=0–3) compounds with different isomeric structures as well as other cage sizes,25 and isolation of Dy2ScN@C80 is quite a tedious process. Therefore, the selective synthesis of Dy2TiC@C80-I, which is isostructural to Dy2ScN@C80-I, opens a more convenient route to EMF-based Dy-SMMs as along as the magnetic properties of the two EMFs are similar.
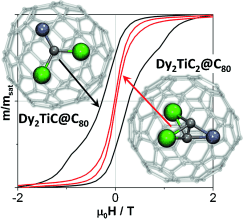
Figure 5 displays the magnetization curve of Dy2TiC@C80-I recorded for different temperatures at a field sweep rate of 5 mT s−1. The system shows magnetic hysteresis with a temperature-dependent opening. The observation indicates that the system exhibits a slow relaxation of the magnetization with a decay rate that depends on the temperature. At a constant temperature, the opening is also determined by the field sweep rate as demonstrated in Figure S9. Similar behavior was observed for Dy2ScN@C80 and is characteristic for SMMs. The stability of the remnant magnetization can be quantified by determining the temperature at which the zero-field magnetization relaxation time is 100 s, the so-called 100 s blocking temperature (TB100).26 In the present case, we obtain TB100=1.7 K for Dy2TiC@C80-I as compared to TB100=3.6 K for Dy2ScN@NC80.12, 26c The higher TB100 value for the nitride clusterfullerene indicates that the nitride ion in the endohedral cluster is advantageous for stronger SMMs.
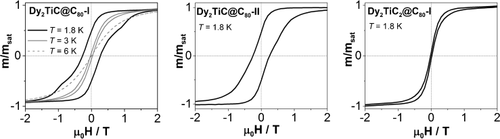
Magnetization curves of Dy2TiC@C80-I, Dy2TiC@C80-II, and Dy2TiC2@C80-I measured by SQUID. All shown curves are measured at 1.8 K with a sweep rate of 5 mT s−1; for Dy2TiC@C80-I the measurements at 3 K and 6 K are also shown.
Isolation of Dy2TiC@C80-II and Dy2TiC2@C80-I allows us to study how the cage isomerism and the carbide cluster composition affect magnetic properties. Corresponding magnetization curves measured at 1.8 K are shown in Figure 5. The two isomers of Dy2TiC@C80 have a similar field dependence and the same TB100 value to within 0.1 K. In contrast, Dy2TiC2@C80 exhibits a narrower hysteresis than the Dy2TiC@C80 isomers. The TB100 value of Dy2TiC2@C80 is below 1.5 K demonstrating that it is the softest magnet in the series. Thus, substitution of a single carbide ion by an acetylide unit in the endohedral cluster has an obvious deleterious effect on the SMM properties.
This work shows that lanthanide–Ti carbide clusterfullerenes can be synthesized with high selectivity using methane as a reactive gas. In particular, Dy2TiC@C80-I is the first mixed-metal EMF obtained as a main fullerene product and separated without elaborate chromatographic procedures. Similar to its Dy2ScN@C80 analogue, Dy2TiC@C80-I is a single-molecule magnet. Thus, the class of EMF-SMMs is expanded to carbide clusterfullerenes. The study of the second isomer of Dy2TiC@C80 showed that the carbon cage isomerism has little effect on the magnetic behavior as the two isomers behave similarly. At the same time, we found a strong dependence of SMM properties on the central nonmetal unit: the strength of Dy2-SMMs is decreases in the series Dy2ScN>Dy2TiC>Dy2TiC2.
Acknowledgements
We are grateful to Christin Scheunert and Pauline Voigt for their help in the synthesis of fullerenes. We acknowledge funding by the DFG (grants PO 1602/1-2 and DU225/31-1), the Swiss National Science Foundation (200021L_147201) within the DACH program, and the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement no. 648295 “GraM3”). Computational resources were provided by the Center for Information Services and High Performance Computing (ZIH) in TU-Dresden.