The Role of Oxygen in the Degradation of Methylammonium Lead Trihalide Perovskite Photoactive Layers†
S.A.H. acknowledges financial support from the Engineering and Physical Sciences Research Council (EPSRC) through EP/H040218/2 and EP/K010298/1 projects. S.A.H. acknowledges financial support from the European Community’s Seventh Framework Programme (Nanomatcell, grant agreement number 308997). T.R. acknowledges financial support from the Austrian Science Fund (FWF) under the grant number J3515-N20.
Graphical Abstract
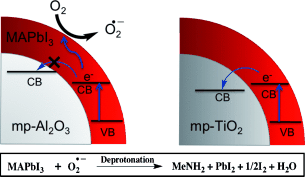
The influence of light and oxygen on the stability of CH3NH3PbI3 perovskite-based photoactive layers is investigated. Upon exposure to both light and dry air, the mesoporous (mp) Al2O3/CH3NH3PbI3 layers decompose to methylamine, PbI2, and I2. This degradation is initiated by the reaction of superoxide (O2−) with the methylammonium moiety of the perovskite absorber. MA=methyl ammonium, CB=conduction band, VB=valence band.
Abstract
In this paper we report on the influence of light and oxygen on the stability of CH3NH3PbI3 perovskite-based photoactive layers. When exposed to both light and dry air the mp-Al2O3/CH3NH3PbI3 photoactive layers rapidly decompose yielding methylamine, PbI2, and I2 as products. We show that this degradation is initiated by the reaction of superoxide (O2−) with the methylammonium moiety of the perovskite absorber. Fluorescent molecular probe studies indicate that the O2− species is generated by the reaction of photoexcited electrons in the perovskite and molecular oxygen. We show that the yield of O2− generation is significantly reduced when the mp-Al2O3 film is replaced with an mp-TiO2 electron extraction and transport layer. The present findings suggest that replacing the methylammonium component in CH3NH3PbI3 to a species without acid protons could improve tolerance to oxygen and enhance stability.