A Triphenylamine with Two Phenoxy Radicals Having Unusual Bonding Patterns and a Closed-Shell Electronic State†
This work was partly supported by a Grant-in-Aid for Young Scientists (B) (26810023) from the Japan Society for the Promotion of Science (JSPS), a Grant-in-Aid for Scientific Research (B) (24310090) from JSPS, and a Grant-in-Aid for Scientific Research on Innovative Areas “New Polymeric Materials Based on Element-Blocks (No. 2401)” (Nos. 24102014 and 25102516) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT). D.S. thanks the JSPS Research Fellowship for Young Scientists.
Graphical Abstract
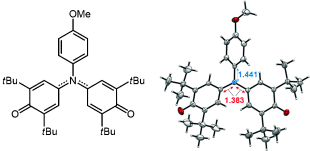
In a (closed) nut shell: A novel triphenylamine derivative having two phenoxy radicals appended to the amino nitrogen atom has been prepared. This molecule has an unexpected closed-shell electronic state, even at room temperature, in spite of its structural similarity to the galvinoxyl radical. The molecule features two CN bonds with multiple-bond character and a remarkably low HOMO–LUMO gap.
Abstract
Reported herein is the structure and the electronic properties of a novel triphenylamine derivative having two phenoxy radicals appended to the amino nitrogen atom. X-ray single crystal analysis and the magnetic resonance measurements demonstrates the unexpected closed-shell electronic structure, even at room temperature, of the molecule and two unusual CN bonds with multiple-bond character. The theoretical calculations support the experimentally determined molecular geometry with the closed-shell electronic structure, and predicted a small HOMO–LUMO gap originating from the nonbonding character of the HOMO. The optical and electrochemical measurements show that the molecule has a remarkably small HOMO–LUMO gap compared with its triphenylamine precursor.