Rhodium-Catalyzed Hydroformylation of 1,1-Disubstituted Allenes Employing the Self-Assembling 6-DPPon System†
Alexander Köpfer
Institut für Organische Chemie, Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg im Breisgau (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Bernhard Breit
Institut für Organische Chemie, Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg im Breisgau (Germany)
Institut für Organische Chemie, Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg im Breisgau (Germany)Search for more papers by this authorAlexander Köpfer
Institut für Organische Chemie, Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg im Breisgau (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Bernhard Breit
Institut für Organische Chemie, Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg im Breisgau (Germany)
Institut für Organische Chemie, Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg im Breisgau (Germany)Search for more papers by this authorThis work was supported by the DFG, the International Research, Training Group “Catalysts and Catalytic Reactions for Organic, Synthesis” (IRTG 1038), the Fonds der Chemischen Industrie, and the Krupp Foundation. We thank Umicore, BASF, and Wacker for generous gifts of chemicals. Sonka Zeyner is acknowledged for highly motivated and skillful technical assistance.
Graphical Abstract
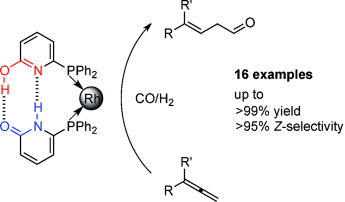
β,γ-Unsaturated aldehydes are obtained by a rhodium-catalyzed hydroformylation of 1,1-disubstituted allenes. For unsymmetrically 1,1-disubstituted allenes the Z-configured product is formed in up to about 95 % selectivity. This is the first time that these building blocks are accessible by hydroformylation of allenes. The utility of this methodology is demonstrated by further transformations of one of the obtained products.
Abstract
A rhodium-catalyzed hydroformylation of 1,1-disubstituted allenes is reported. Using a RhI/6-DPPon catalyst system, one can obtain β,γ-unsaturated aldehydes in high regio- and chemoselectivity. The Z-configured product is formed with up to >95 % selectivity when unsymmetrically 1,1-disubstituted allenes are submitted to the reaction conditions. This is the first time that these interesting building blocks are accessible by hydroformylation of allenes. The utility of this methodology is demonstrated by further transformations of one of the obtained products.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201502086_sm_miscellaneous_information.pdf4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aK. Weissermel, H. J. Arpe, Industrial Organic Chemistry, Wiley-VCH, Weinheim, 2003, pp. 127–141;
10.1002/9783527619191.ch6 Google Scholar
- 1b Rhodium Catalyzed Hydroformylation (Eds.: ), Kluwer, Dordrecht, 2000;
- 1cB. Breit, W. Seiche, Synthesis 2001, 1–36;
- 1dR. Franke, D. Selent, A. Börner, Chem. Rev. 2012, 112, 5675–5732.
- 2S. Naqvi, Oxo Alcohols. Process Economics Program Report 21E, SRI Consulting, Menlo Park, CA, 2010.
- 3
- 3aK. Doyama, T. Joh, S. Takahashi, T. Shiohara, Tetrahedron Lett. 1986, 27, 4497–4500;
- 3bJ. R. Johnson, G. D. Cuny, S. L. Buchwald, Angew. Chem. Int. Ed. Engl. 1995, 34, 1760–1761; Angew. Chem. 1995, 107, 1877–1879;
- 3cY. Ishii, K. Miyashita, K. Kamita, M. J. Hidai, J. Am. Chem. Soc. 1997, 119, 6448–6449;
- 3dB. G. Van den Hoven, H. J. Alper, Org. Chem. 1999, 64, 3964–3968;
- 3eB. G. Van den Hoven, H. J. Alper, Org. Chem. 1999, 64, 9640–9645;
- 3fV. Agabekov, W. Seiche, B. Breit, Chem. Sci. 2013, 4, 2418–2422;
- 3gX. Fang, M. Zhang, R. Jackstell, M. Beller, Angew. Chem. Int. Ed. 2013, 52, 4645–4649; Angew. Chem. 2013, 125, 4743–4747;
- 3hX. Fang, R. Jackstell, M. Beller, Chem. Eur. J. 2014, 20, 7939–7942.
- 4
- 4aW. H. Clement, M. Orchin, Ind. Eng. Chem. Prod. Res. Dev. 1965, 4, 283–286;
- 4bB. Fell, H. Bahrmann, J. Mol. Catal. 1977, 2, 211–218;
- 4cH. Bahrmann, B. Fell, J. Mol. Catal. 1980, 8, 329–337;
- 4dC. Botteghi, M. Branca, A. Saba, J. Organomet. Chem. 1980, 184, C 17–C19;
- 4eP. W. N. M. van Leeuwen, C. F. Roobeek, J. Mol. Catal. 1985, 31, 345–353;
- 4fJ. C. Chalchat, R. P. Garry, E. Lecomte, A. Michet, Flavour Fragrance J. 1991, 6, 179–182;
- 4gS. Bertozzi, N. Campigli, G. Vitulli, R. Lazzaroni, P. Salvadori, J. Organomet. Chem. 1995, 487, 41–45;
- 4hT. Horiuchi, T. Ohta, K. Nozaki, H. Takaya, Chem. Commun. 1996, 155–156;
- 4iT. Horiuchi, T. Ohta, E. Shirakawa, K. Nozaki, H. Takaya, Tetrahedron 1997, 53, 7795–7804;
- 4jP. Liu, E. N. Jacobsen, J. Am. Chem. Soc. 2001, 123, 10772–10773;
- 4kH. J. V. Barros, B. E. Hanson, E. N. dos Santos, E. V. Gusevskaya, Appl. Catal. A 2004, 278, 57–63;
- 4lH. J. V. Barros, J. G. da Silva, C. C. Guimarães, E. N. dos Santos, E. V. Gusevskaya, Organometallics 2008, 27, 4523–4531;
- 4mS. E. Smith, T. Rosendahl, P. Hofmann, Organometallics 2011, 30, 3643–3651;
- 4nA. L. Watkins, C. R. Landis, Org. Lett. 2011, 13, 164–167;
- 4oT. E. Smith, S. J. Fink, Z. G. Levine, K. A. McClelland, A. A. Zackheim, M. E. Daub, Org. Lett. 2012, 14, 1452–1455.
- 5
- 5aB. Fell, M. Beutler, Erdöl Kohle Erdgas 1976, 29, 149–153;
- 5bC.-F. Huo, Y.-W. Li, M. Beller, H. Jiao, Chem. Eur. J. 2005, 11, 889–902;
- 5cH. Guo, S. Ma, Adv. Synth. Catal. 2008, 350, 1213–1217.
- 6
- 6aB. Sam, B. Breit, M. J. Krische, Angew. Chem. Int. Ed. 2015, 54, 3267–3274; Angew. Chem. 2015, 127, 3317–3325;
- 6bC. C. Bausch, R. L. Patman, B. Breit, M. J. Krische, Angew. Chem. Int. Ed. 2011, 50, 5687–5690; Angew. Chem. 2011, 123, 5805–5808;
- 6cA. Köpfer, B. Sam, B. Breit, M. J. Krische, Chem. Sci. 2013, 4, 1876–1880;
- 6dM.-Y. Ngai, E. Skucas, M. J. Krische, Org. Lett. 2008, 10, 2705–2708;
- 6eB. Sam, T. P. Montgomery, M. J. Krische, Org. Lett. 2013, 15, 3790–3793.
- 7B. M. Trost, Science 1991, 254, 1471–1477.
- 8
- 8aS.-S. Ng, T. F. Jamison, J. Am. Chem. Soc. 2005, 127, 7320–7321;
- 8bS.-S. Ng, T. F. Jamison, Tetrahedron 2005, 61, 11405–11417;
- 8cS.-S. Ng, T. F. Jamison, Tetrahedron 2006, 62, 11350–11359.
- 96-DPPon is commercially available (Sigma Aldrich);
- 9aB. Breit, W. Seiche, J. Am. Chem. Soc. 2003, 125, 6608–6609;
- 9bB. Breit, Angew. Chem. Int. Ed. 2005, 44, 6816–6825; Angew. Chem. 2005, 117, 6976–6986;
- 9cW. Seiche, A. Schuschkowski, B. Breit, Adv. Synth. Catal. 2005, 347, 1488–1494;
- 9dU. Gellrich, W. Seiche, M. Keller, B. Breit, Angew. Chem. Int. Ed. 2012, 51, 11033–11038; Angew. Chem. 2012, 124, 11195–11200;
- 9eU. Gellrich, D. Himmel, M. Meuwly, B. Breit, Chem. Eur. J. 2013, 19, 16272–16281.
- 10
- 10aJ. Meeuwissen, J. N. H. Reek, Nat. Chem. 2010, 2, 615–621;
- 10bM. J. Wilkinson, P. W. N. M. van Leeuwen, J. N. H. Reek, Org. Biomol. Chem. 2005, 3, 2371–2383;
- 10cS. A. Moteki, K. Toyama, Z. Liu, J. Ma, A. Holmes, J. M. Takacs, Chem. Commun. 2012, 48, 263–265.
- 11C. Waloch, J. Wieland, M. Keller, B. Breit, Angew. Chem. Int. Ed. 2007, 46, 3037–3039; Angew. Chem. 2007, 119, 3097–3099.
- 12The hydroformylation of differently substituted allenes was not as successful under these conditions: 5-Phenyl-1,2-pentadiene was converted to a complex mixture of more than five aldehydes (32 % selectivity for the β,γ-unsaturated aldehyde as the main product, 13 % selectivity for the fully saturated linear aldehyde); as an example for an internal allene, 1,7-diphenylhepta-3,4-diene could be reisolated quantitatively.
- 13
- 13aL. A. van der Veen, P. C. J. Kamer, P. W. N. M. van Leeuwen, Angew. Chem. Int. Ed. 1999, 38, 336–338;
10.1002/(SICI)1521-3773(19990201)38:3<336::AID-ANIE336>3.0.CO;2-P CAS PubMed Web of Science® Google ScholarAngew. Chem. 1999, 111, 349–351;
- 13bH. Klein, R. Jackstell, K.-D. Wiese, C. Borgmann, M. Beller, Angew. Chem. Int. Ed. 2001, 40, 3408–3411;
10.1002/1521-3773(20010917)40:18<3408::AID-ANIE3408>3.0.CO;2-A CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 3505–3508;
- 13cD. Selent, D. Hess, K.-D. Wiese, D. Röttger, C. Kunze, A. Börner, Angew. Chem. Int. Ed. 2001, 40, 1696–1698;
10.1002/1521-3773(20010504)40:9<1696::AID-ANIE16960>3.0.CO;2-2 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 1739–1741;
- 13dR. P. J. Bronger, P. C. J. Kamer, P. W. N. M. van Leeuwen, Organometallics 2003, 22, 5358–5369;
- 13eR. P. J. Bronger, J. P. Bermon, J. Herwig, P. C. J. Kamer, P. W. N. M. van Leeuwen, Adv. Synth. Catal. 2004, 346, 789–799;
- 13fH. Klein, R. Jackstell, M. Beller, Chem. Commun. 2005, 2283–2285;
- 13gY. Yan, X. Zhang, X. Zhang, J. Am. Chem. Soc. 2006, 128, 16058–16061;
- 13hS. Yu, Y. M. Chie, Z. H. Guan, X. Zhang, Org. Lett. 2008, 10, 3469–3472;
- 13iM. A. N. Carvajal, S. Kozuch, S. Shaik, Organometallics 2009, 28, 3656–3665.
- 142 l and 2 o were formed with 80 % selectivity for the desired aldehyde. During isolation some of the material was lost due to the acidity of the CH2-group in α-position of the aldehyde.
- 15The reason for this switch in stereoselectivity is not clear yet, but one could propose that the electronic properties of the silicon substituents might accelerate isomerization of the complex HMd thereby enabling generation of the thermodynamically more favored E-aldehyde.
- 16
- 16aB. M. Trost, M. R. Machacek, Z. Ball, Org. Lett. 2003, 5, 1895–1898;
- 16bS. E. Denmark, S. A. Tymonko, J. Am. Chem. Soc. 2005, 127, 8004–8005.
- 17S. Song, S.-F. Zhu, S. Yang, S. Li, Q.-L. Zhou, Angew. Chem. Int. Ed. 2012, 51, 2708–2711; Angew. Chem. 2012, 124, 2762–2765.
- 18T. S. Mei, H. H. Patel, M. S. Sigman, Nature 2014, 508, 340–344.
- 19M. Petrzilka, J. I. Grayson, Synthesis 1981, 753–786.
- 20T. Mukaiyama, A. Ishida, Chem. Lett. 1975, 319–322.
- 21L. A. Paquette, Tetrahedron 1997, 53, 13971–14020.