Controlling the Selectivity of the Surface Plasmon Resonance Mediated Oxidation of p-Aminothiophenol on Au Nanoparticles by Charge Transfer from UV-excited TiO2†
This work was supported by the Fundação de Amparo à Pesquisa do Estado de Sao Paulo (FAPESP) (grant number 2013/19861-6) and the Conselho Nacional de Desenvolvimento Cientifíco e Tecnologico (CNPq) (grant number 471245/2012-7). P.H.C.C. and R.A.A. thank the CNPq for research fellowships. J.W. thanks FAPESP (grant number 2013/05709-8) for a fellowship.
Graphical Abstract
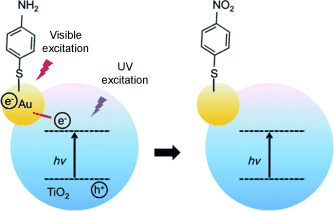
Controlling selectivity: The selectivity of the surface plasmon resonance (SPR) mediated oxidation of p-aminothiophenol was controlled by the choice of catalysts (Au or TiO2–Au nanoparticles; NPs) and by the modulation of the charge transfer from UV-excited TiO2 to Au. While p,p-dimercaptobenzene was obtained using Au NPs as catalyst, the use of TiO2–Au NPs under both UV illumination and SPR excitation led to the formation of p-nitrophenol.
Abstract
Although catalytic processes mediated by surface plasmon resonance (SPR) excitation have emerged as a new frontier in catalysis, the selectivity of these processes remains poorly understood. Here, the selectivity of the SPR-mediated oxidation of p-aminothiophenol (PATP) employing Au NPs as catalysts was controlled by the choice of catalysts (Au or TiO2-Au NPs) and by the modulation of the charge transfer from UV-excited TiO2 to Au. When Au NPs were employed as catalyst, the SPR-mediated oxidation of PATP yielded p,p-dimercaptobenzene (DMAB). When TiO2-Au NPs were employed as catalysts under both UV illumination and SPR excitation, p-nitrophenol (PNTP) was formed from PATP in a single step. Interestingly, PNTP molecules were further reduced to DMAB after the UV illumination was removed. Our data show that control over charge-transfer processes may play an important role to tune activity, product formation, and selectivity in SPR-mediated catalytic processes.