Michael Addition Catalyzed by Chiral Secondary Amine Phosphoramide Using Fluorinated Silyl Enol Ethers: Formation of Quaternary Carbon Stereocenters†
Jin-Sheng Yu
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, 3663N, Zhongshan Road, Shanghai 200062 (China)
Search for more papers by this authorFu-Min Liao
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, 3663N, Zhongshan Road, Shanghai 200062 (China)
Search for more papers by this authorWei-Ming Gao
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, 3663N, Zhongshan Road, Shanghai 200062 (China)
Search for more papers by this authorKui Liao
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, 3663N, Zhongshan Road, Shanghai 200062 (China)
Search for more papers by this authorRun-Lin Zuo
No 15 class of Grade 2012, Luzhou High School, 33 Zhongshan Road, Luzhou 646000 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jian Zhou
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, 3663N, Zhongshan Road, Shanghai 200062 (China)
State Key Laboratory of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (P.R. China)
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, 3663N, Zhongshan Road, Shanghai 200062 (China)Search for more papers by this authorJin-Sheng Yu
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, 3663N, Zhongshan Road, Shanghai 200062 (China)
Search for more papers by this authorFu-Min Liao
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, 3663N, Zhongshan Road, Shanghai 200062 (China)
Search for more papers by this authorWei-Ming Gao
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, 3663N, Zhongshan Road, Shanghai 200062 (China)
Search for more papers by this authorKui Liao
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, 3663N, Zhongshan Road, Shanghai 200062 (China)
Search for more papers by this authorRun-Lin Zuo
No 15 class of Grade 2012, Luzhou High School, 33 Zhongshan Road, Luzhou 646000 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jian Zhou
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, 3663N, Zhongshan Road, Shanghai 200062 (China)
State Key Laboratory of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (P.R. China)
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, 3663N, Zhongshan Road, Shanghai 200062 (China)Search for more papers by this authorWe acknowledge financial support from the 973 program (2011CB808600), NSFC (21222204, 21472049), Ministry of Education (NCET-11-0147 and PCSIRT), and Program of SSCS (13XD1401600).
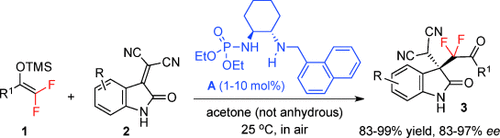
Graphical Abstract
Four C′s: The chiral secondary amine phosphoramide A was developed and serves as a powerful catalyst for the Michael addition of fluorinated enol silyl ethers to tetrasubstituted olefins. The resulting products are obtained with high enantioselectivities and contain a quaternary carbon stereocenter bearing either a difluoroalkyl or monofluoroalkyl group. TMS=trimethylsilyl.
Abstract
A chiral secondary amine phosphoramide was developed and identified as a powerful catalyst for the Mukaiyama–Michael addition of fluorinated enol silyl ethers to tetrasubstituted olefins. The resulting products are obtained with high enantioselectivities and contain a quaternary carbon stereocenter bearing either a difluoroalkyl or monofluoroalkyl group.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201501747_sm_miscellaneous_information.pdf12.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Berkessel, H. Gröger, Asymmetric Organocatalysis, Wiley-VCH, Weinheim, 2005;
- 1bS. Mukherjee, J. W. Yang, S. Hoffmann, B. List, Chem. Rev. 2007, 107, 5471;
- 1cA. Erkkilä, I. Majander, P. M. Pihko, Chem. Rev. 2007, 107, 5416;
- 1dT. D. Beeson, A. Mastracchio, J.-B. Hong, K. Ashton, D. W. C. MacMillan, Science 2007, 316, 582.
- 2
- 2aB. Han, Q.-P. Liu, R. Li, X. Tian, X.-F. Xiong, J.-G. Deng, Y.-C. Chen, Chem. Eur. J. 2008, 14, 8094;
- 2bS. Belot, A. Quintard, N. Krause, A. Alexakis, Adv. Synth. Catal. 2010, 352, 667;
- 2cC. Palumbo, G. Mazzeo, A. Mazziotta, A. Gambacorta, M. A. Loreto, A. Migliorini, S. Superchi, D. Tofani, T. Gasperi, Org. Lett. 2011, 13, 6248;
- 2dA. Lattanzi, C. D. Fusco, A. Russo, A. Poater, L. Cavallo, Chem. Commun. 2012, 48, 1650;
- 2eC. Wang, X. Yang, D. Enders, Chem. Eur. J. 2012, 18, 4832.
- 3Only one example using achiral secondary amine to activate TMSCN for cyanosilylation of aldehydes is reported: S. Kobayasi, Y. Tsuchiya, T. Mukaiyama, Chem. Lett. 1991, 537.
- 4
- 4aJ. Gawronski, N. Wascinska, J. Gajewy, Chem. Rev. 2008, 108, 5227;
- 4bP. L. Fuchs, Handbook of Reagents for Organic Synthesis, Reagents for Silicon-Mediated Organic Synthesis, Wiley, Chichester, 2011.
- 5For reviews, see:
- 5aS. E. Denmark, G. L. Beutner, Angew. Chem. Int. Ed. 2008, 47, 1560; Angew. Chem. 2008, 120, 1584;
- 5bM. J. Gaunt, C. C. C. Johansson, Chem. Rev. 2007, 107, 5596;
- 5cG. C. Fu, Acc. Chem. Res. 2004, 37, 542;
- 5dS.-K. Tian, Y. Chen, J. Hang, L. Tang, P. McDaid, L. Deng, Acc. Chem. Res. 2004, 37, 621.
- 6aS.-K. Tian, R. Hong, L. Deng, J. Am. Chem. Soc. 2003, 125, 9900;
- 6bS. E. Denmark, W.-J. Chung, J. Org. Chem. 2006, 71, 4002; For a review:
- 6cW. Wang, X. Liu, L. Lin, X. Feng, Eur. J. Org. Chem. 2010, 4751.
- 7
- 7aD. Enders, K. Gottfried, G. Raabe, Adv. Synth. Catal. 2010, 352, 3147;
- 7bY.-L. Liu, F. Zhou, J.-J. Cao, C.-B. Ji, M. Ding, J. Zhou, Org. Biomol. Chem. 2010, 8, 3847;
- 7cY.-L. Liu, T.-D. Shi, F. Zhou, X.-L. Zhao, X. Wang, J. Zhou, Org. Lett. 2011, 13, 3826;
- 7dY.-L. Liu, J. Zhou, Chem. Commun. 2013, 49, 4421;
- 7eF.-G. Zhang, X.-Y. Zhu, S. Li, J. Nie, J.-A. Ma, Chem. Commun. 2012, 48, 11552;
- 7fH. Xie, A. Song, X. Song, X. Zhang, W. Wang, Tetrahedron Lett. 2013, 54, 1409;
- 7gN.-K. Li, Z.-M. Liu, X.-F. Huang, J.-X. Zhang, X. Chen, Y. Wang, X.-W. Wang, RSC Adv. 2013, 3, 9154;
- 7hH.-X. He, D.-M. Du, Eur. J. Org. Chem. 2014, 6190; For a review:
- 7iY.-L. Liu, J. Zhou, Synthesis 2015, 1210.
- 8
- 8aR. P. Singh, B. M. Foxman, L. Deng, J. Am. Chem. Soc. 2010, 132, 9558;
- 8bY.-L. Liu, J. Zhou, Chem. Commun. 2012, 48, 1919;
- 8cY.-L. Liu, F.-M. Liao, Y.-F. Niu, X.-L. Zhao, J. Zhou, Org. Chem. Front. 2014, 1, 742;
- 8dY.-L. Liu, J. Zhou, Acta Chim. Sin. 2012, 70, 1451.
- 9
- 9aJ.-S. Yu, Y.-L. Liu, J. Tang, X. Wang, J. Zhou, Angew. Chem. Int. Ed. 2014, 53, 9512; Angew. Chem. 2014, 126, 9666;
- 9bJ.-J. Cao, F. Zhou, J. Zhou, Angew. Chem. Int. Ed. 2010, 49, 4976; Angew. Chem. 2010, 122, 5096;
- 9cZ.-Y. Cao, Y. Zhang, C.-B. Ji, J. Zhou, Org. Lett. 2011, 13, 6398.
- 10J. Zhou, Multicatalyst system in asymmetric catalysis, Wiley, New York, 2014, Chap. 3.
10.1002/9781118846919 Google Scholar
- 11
- 11aM. Ding, F. Zhou, Y.-L. Liu, C.-H. Wang, X.-L. Zhao, J. Zhou, Chem. Sci. 2011, 2, 2035;
- 11bW.-M. Gao, J.-S. Yu, Y.-L. Zhao, Y.-L. Liu, F. Zhou, H.-H. Wu, J. Zhou, Chem. Commun. 2014, 50, 15179.
- 12
- 12aS. E. Denmark, T. Wynn, J. Am. Chem. Soc. 2001, 123, 6199;
- 12bS. E. Denmark, T. Wynn, G. L. Beutner, J. Am. Chem. Soc. 2002, 124, 13405;
- 12cS. E. Denmark, R. A. Stavenger, Acc. Chem. Res. 2000, 33, 432.
- 13
- 13aB. Qin, X. H. Liu, J. Shi, K. Zheng, H. T. Zhao, X. M. Feng, J. Org. Chem. 2007, 72, 2374;
- 13bX. H. Liu, L. L. Lin, X. M. Feng, Acc. Chem. Res. 2011, 44, 574.
- 14For organocatalytic versions:
- 14aS. P. Brown, N. C. Goodwin, D. W. C. MacMillan, J. Am. Chem. Soc. 2003, 125, 1192;
- 14bV. Gupta, S. Sudhir V, T. Mandal, C. Schneider, Angew. Chem. Int. Ed. 2012, 51, 12609; Angew. Chem. 2012, 124, 12778;
- 14cE. K. Kemppainen, G. Sahoo, A. Piisola, A. Hamza, B. Kótai, I. Pápai, P. M. Pihko, Chem. Eur. J. 2014, 20, 5983;
- 14dF.-Y. Zhang, E. J. Corey, Org. Lett. 2001, 3, 639;
- 14eW. Wang, H. Li, J. Wang, Org. Lett. 2005, 7, 1637; Chiral metal catalyzed version:
- 14fN. Takenaka, J. P. Abell, H. Yamamoto, J. Am. Chem. Soc. 2007, 129, 742;
- 14gX. Xu, W.-H. Hu, M. P. Doyle, Angew. Chem. Int. Ed. 2011, 50, 6392; Angew. Chem. 2011, 123, 6516;
- 14hD. A. Evans, T. Rovis, M. C. Kozlowski, J. S. Tedrow, J. Am. Chem. Soc. 1999, 121, 1994; For a review:
- 14iG. Casiraghi, L. Battistini, C. Curti, G. Rassu, F. Zanardi, Chem. Rev. 2011, 111, 3076.
- 15
- 15aG. K. S. Prakash, P. Beier, Angew. Chem. Int. Ed. 2006, 45, 2172; Angew. Chem. 2006, 118, 2228;
- 15bV. A. Brunet, D. O′Hagan, Angew. Chem. Int. Ed. 2008, 47, 1179; Angew. Chem. 2008, 120, 1198.
- 16É. Bélanger, K. Cantin, O. Messe, M. Tremblay, J.-F. Paquin, J. Am. Chem. Soc. 2007, 129, 1034.
- 17
- 17aW. Kashikura, K. Mori, T. Akiyama, Org. Lett. 2011, 13, 1860;
- 17bZ. Yuan, L. Mei, Y. Wei, M. Shi, P. V. Kattamuri, P. McDowell, G. Li, Org. Biomol. Chem. 2012, 10, 2509–2513.
- 18
- 18aJ. T. Mohr, M. R. Krout, B. M. Stoltz, Nature 2008, 455, 323;
- 18bB. M. Trost, C. Jiang, Synthesis 2006, 369.
- 19
- 19aZ.-Y. Cao, X. Wang, C. Tan, X.-L. Zhao, J. Zhou, K. Ding, J. Am. Chem. Soc. 2013, 135, 8197;
- 19bF. Zhou, C. Tan, J. Tang, Y.-Y. Zhang, W.-M. Gao, H.-H. Wu, Y.-H. Yu, J. Zhou, J. Am. Chem. Soc. 2013, 135, 10994;
- 19cY.-L. Liu, B.-L. Wang, J.-J. Cao, L. Chen, Y.-X. Zhang, C. Wang, J. Zhou, J. Am. Chem. Soc. 2010, 132, 15176;
- 19dY.-L. Liu, X. Wang, Y.-L. Zhao, F. Zhu, X.-P. Zeng, L. Chen, C.-H. Wang, X.-L. Zhao, J. Zhou, Angew. Chem. Int. Ed. 2013, 52, 13735; Angew. Chem. 2013, 125, 13980.
- 20aY.-B. Lan, H. Zhao, Z.-M. Liu, G.-G. Liu, J.-C. Tao, X.-W. Wang, Org. Lett. 2011, 13, 4866;
- 20bW.-B. Chen, Z.-J. Wu, Q.-L. Pei, L.-F. Cun, X.-M. Zhang, W.-C. Yuan, Org. Lett. 2010, 12, 3132;
- 20cF. Zhong, X. Han, Y. Wang, Y. Lu, Angew. Chem. Int. Ed. 2011, 50, 7837; Angew. Chem. 2011, 123, 7983;
- 20dL. Liu, D. Wu, S. Zheng, T. Li, X. Li, S. Wang, J. Li, H. Li, W. Wang, Org. Lett. 2012, 14, 134.
- 21For reviews:
- 21aX. Yang, T. Wu, R. J. Phipps, F. D. Toste, Chem. Rev. 2015, 115, 826;
- 21bJ.-H. Lin, J.-C. Xiao, Tetrahedron Lett. 2014, 55, 6147;
- 21cY.-L. Liu, J.-S. Yu, J. Zhou, Asian J. Org. Chem. 2013, 2, 194;
- 21dZ. He, Y. Huang, F. Verpoort, Acta Chim. Sin. 2013, 71, 700;
- 21eG. Valero, X. Companyó, R. Rios, Chem. Eur. J. 2011, 17, 2018;
- 21fJ. Hu, W. Zhang, F. Wang, Chem. Commun. 2009, 7465;
- 21gN. Shibata, S. Mizuta, H. Kawai, Tetrahedron: Asymmetry 2008, 19, 2633.
- 22For phosphoramide-based chiral ligands or organocatalysts:
- 22aB. Burns, J. R. Studley, M. Wills, Tetrahedron Lett. 1993, 34, 7105;
- 22bM. Shi, C.-J. Wang, Adv. Synth. Catal. 2003, 345, 971;
- 22cD. A. Watson, M. Chiu, R. G. Bergman, Organometallics 2006, 25, 4731;
- 22dD. J. Morris, A. S. Partridge, C. V. Manville, D. T. Racys, G. Woodward, G. Docherty, M. Wills, Tetrahedron Lett. 2010, 51, 209;
- 22eA. Lu, R. Wu, Y. Wang, Z. Zhou, G. Wu, J. Fang, C. Tang, Eur. J. Org. Chem. 2010, 2057;
- 22fS. Vellalath, I. Čorić, B. List, Angew. Chem. Int. Ed. 2010, 49, 9749; Angew. Chem. 2010, 122, 9943;
- 22gH. Y. Huang, H. Zong, B. Shen, H. F. Yue, G. L. Bian, L. Song, Tetrahedron 2014, 70, 1289.
- 23
- 23aS. Ogawa, N. Shibata, J. Inagaki, S. Nakamura, T. Toru, M. Shiro, Angew. Chem. Int. Ed. 2007, 46, 8666; Angew. Chem. 2007, 119, 8820;
- 23bY. Hamashima, T. Suzuki, H. Takano, Y. Shimura, M. Sodeoka, J. Am. Chem. Soc. 2005, 127, 10164;
- 23cN. Shibata, J. Kohno, K. Takai, T. Ishimaru, S. Nakamura, T. Toru, S. Kanemasa, Angew. Chem. Int. Ed. 2005, 44, 4204; Angew. Chem. 2005, 117, 4276;
- 23dS. Ogawa, N. Iida, E. Tokunaga, M. Shiro, N. Shibata, Chem. Eur. J. 2010, 16, 7090;
- 23eL. Wu, L. Falivene, E. Drinkel, S. Grant, A. Linden, L. Cavallo, R. Dorta, Angew. Chem. Int. Ed. 2012, 51, 2870; Angew. Chem. 2012, 124, 2924;
- 23fN.-J. Zhong, F. Wei, Q.-Q. Xuan, L. Liu, D. Wang, Y.-J. Chen, Chem. Commun. 2013, 49, 11071;
- 23gI. Saidalimu, X. Fang, X.-P. He, J. Liang, X. Yang, F. Wu, Angew. Chem. Int. Ed. 2013, 52, 5566; Angew. Chem. 2013, 125, 5676;
- 23hC. Wu, G. Li, W. Sun, M. Zhang, L. Hong, R. Wang, Org. Lett. 2014, 16, 1960.
- 24
- 24aF. Zhou, Y.-L. Liu, J. Zhou, Adv. Synth. Catal. 2010, 352, 1381;
- 24bK. Shen, X. Liu, L. Lin, X. Feng, Chem. Sci. 2012, 3, 327;
- 24cR. Dalpozzo, G. Bartoli, G. Bencivenni, Chem. Soc. Rev. 2012, 41, 7247; For recent examples:
- 24dZ. Zhang, W. Zheng, J. C. Antilla, Angew. Chem. Int. Ed. 2011, 50, 1135; Angew. Chem. 2011, 123, 1167;
- 24eK. Ohmatsu, M. Kiyokawa, T. Ooi, J. Am. Chem. Soc. 2011, 133, 1307;
- 24fK. Zheng, C. Yin, X. Liu, L. Lin, X. Feng, Angew. Chem. Int. Ed. 2011, 50, 2573; Angew. Chem. 2011, 123, 2621;
- 24gC. Guo, J. Song, J.-Z. Huang, P.-H. Chen, S.-W. Luo, L.-Z. Gong, Angew. Chem. Int. Ed. 2012, 51, 1046; Angew. Chem. 2012, 124, 1070;
- 24hF. Manoni, S. J. Connon, Angew. Chem. Int. Ed. 2014, 53, 2628; Angew. Chem. 2014, 126, 2666;
- 24iB. Xiang, K. M. Belyk, R. A. Reamer, N. Yasuda, Angew. Chem. Int. Ed. 2014, 53, 8375; Angew. Chem. 2014, 126, 8515;
- 24jJ. Stiller, P. H. Poulsen, D. C. Cruz, J. Dourado, R. L. Davis, K. A. Jørgensen, Chem. Sci. 2014, 5, 2052;
- 24kF. Shi, R.-Y. Zhu, W. Dai, C.-S. Wang, S.-J. Tu, Chem. Eur. J. 2014, 20, 2597.
- 25
- 25aI. Sharma, D. S. Tan, Nat. Chem. 2013, 5, 157;
- 25bY. Zheng, C. M. Tice, S. B. Singh, Bioorg. Med. Chem. Lett. 2014, 24, 3673.
- 26It was not clear now whether the phosphoramide moiety of C1 served as a hydrogen-bond donor or as a Lewis base, as the N-methylated catalyst C9 achieved comparable reactivity and ee value in the reaction of 1 a and 2 a.
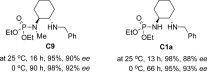
- 27CCDC 1047041 (5 b), CCDC 1047043 (6), CCDC 1047044 (10), and CCDC 1047042 (12) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.