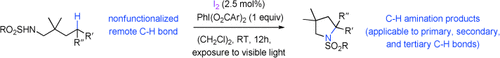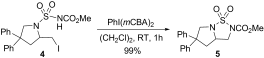An Iodine-Catalyzed Hofmann–Löffler Reaction†
We thank Prof. Dr. J. M. González and Prof. Dr. P. Melchiorre for helpful discussions and A. Bahamonde for support in the quantum yield determination. Financial support of this project was provided by the Cellex-ICIQ Programme (postdoctoral contract to C. M.) and the Spanish Ministry for Economy and Competitiveness (CTQ2011-25027 and CTQ2013-50105-EXP grants to K. M., and Severo Ochoa Excellence Accreditation 2014–2018 to ICIQ, SEV-2013-0319).
Graphical Abstract
Iodine does it! The first catalytic Hofmann–Löffler reaction proceeds with a combination of catalytic amounts of molecular iodine and a modified hypervalent iodine(III) reagent. The reaction proceeds under mild catalytic conditions and the intramolecular CH amination addresses primary, secondary, and tertiary CH groups.
Abstract
Iodine reagents have been identified as economically and ecologically benign alternatives to transition metals, although their application as molecular catalysts in challenging CH oxidation reactions has remained elusive. An attractive iodine oxidation catalysis is now shown to promote the convenient conversion of carbon–hydrogen bonds into carbon–nitrogen bonds with unprecedented complete selectivity. The reaction proceeds by two interlocked catalytic cycles comprising a radical chain reaction, which is initiated by visible light as energy source. This unorthodox synthetic strategy for the direct oxidative amination of alkyl groups has no biosynthetic precedence and provides an efficient and straightforward access to a general class of saturated nitrogenated heterocycles.