Temperature-Controlled Bidirectional Enantioselectivity in a Dynamic Catalyst for Asymmetric Hydrogenation†
This work was supported by the European Research Council under Grant Agreements No. StG 258740. G.S. acknowledges support from the Fonds der Chemischen Industrie for a PhD fellowship. We thank Dr. F. Rominger for X-ray structure analysis.
Graphical Abstract
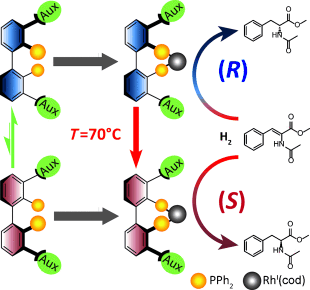
Temperature switches enantioselectivity! A stereochemically flexible diastereomeric rhodium(I) catalyst has been designed for the asymmetric hydrogenation of prochiral (Z)-α-acetamidocinnamates and α-substituted acrylates. The catalyst changes its enantioselectivity depending on the temperature to produce each single enantiomer in high yield and a constant high enantioselectivity.
Abstract
Asymmetric catalysis using enantiomerically pure catalysts is one of the most widely used methods for the preparation of enantiomerically pure compounds. The separate synthesis of both enantiomerically pure compounds requires tedious and time-consuming preparation of both enantiomerically pure catalysts or chiral separation of the racemic products. Here, we report a stereochemically flexible diastereomeric rhodium(I) catalyst for asymmetric hydrogenations of prochiral (Z)-α-acetamidocinnamates and α-substituted acrylates, which changes its enantioselectivity depending on the temperature to produce each enantiomerically pure compound in high yield with constant high enantioselectivity over time. The same axially chiral rhodium(I) catalyst produces (R)-phenylalanine derivatives in enantiomeric ratios of up to 87:13 (R/S) at low temperature and up to 3:97 (R/S) of the corresponding S enantiomers after re-equilibration of the same catalyst at elevated temperature.