Catalytic Asymmetric Total Synthesis of (−)-Galanthamine and (−)-Lycoramine†
Lei Li
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China)
These authors contributed equally to this work.
Search for more papers by this authorQiao Yang
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China)
These authors contributed equally to this work.
Search for more papers by this authorDr. Yuan Wang
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China)
Search for more papers by this authorCorresponding Author
Prof. Yanxing Jia
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China)
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China)Search for more papers by this authorLei Li
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China)
These authors contributed equally to this work.
Search for more papers by this authorQiao Yang
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China)
These authors contributed equally to this work.
Search for more papers by this authorDr. Yuan Wang
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China)
Search for more papers by this authorCorresponding Author
Prof. Yanxing Jia
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China)
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China)Search for more papers by this authorWe are grateful to the National Natural Science Foundation of China (Nos. 21402003, and 21290183) for their financial support.
Graphical Abstract
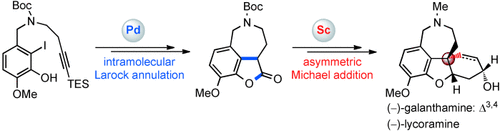
How gallant: The catalytic asymmetric total synthesis of both (−)-galanthamine and (−)-lycoramine were achieved by using a conceptually new strategy. Two metal-catalyzed reactions were used as the key steps, and include a palladium-catalyzed intramolecular Larock annulation reaction and enantioselective conjugate addition reaction catalyzed by a ScIII/N,N′-dioxide complex. Boc=tert-butoxycarbonyl, TES=triethylsilyl.
Abstract
The catalytic asymmetric total syntheses of (−)-galanthamine (1) and (−)-lycoramine (2) have been achieved by using a conceptually new strategy featuring two metal-catalyzed reactions as the key steps. A new method for the construction of 3,4-fused benzofurans has been developed through a palladium-catalyzed intramolecular Larock annulation reaction, which was successfully applied to the construction of the ABD tricyclic skeleton of 1 and 2. To achieve the asymmetric synthesis of 1 and 2, a ScIII/N,N′-dioxide complex was used to catalyze the enantioselective conjugate addition of 3-alkyl-substituted benzofuranone to methyl vinyl ketone for the construction of a chiral quaternary carbon center.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201411338_sm_miscellaneous_information.pdf5.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For a recent review, see:
- 1aU. Rinner, T. Hudlicky, Top. Curr. Chem. 2012, 309, 33; for recent syntheses reported after those cited in Ref. [1a], see:
- 1bM. Ichiki, H. Tanimoto, S. Miwa, R. Saito, T. Sato, N. Chida, Chem. Eur. J. 2013, 19, 264;
- 1cJ. Li, G.-L. Liu, X.-H. Zhao, J.-Y. Du, H. Qu, W.-D. Chu, M. Ding, C.-Y. Jin, M.-X. Wei, C.-A. Fan, Chem. Asian J. 2013, 8, 1105;
- 1dV. Varghese, T. Hudlicky, Angew. Chem. Int. Ed. 2014, 53, 4355; Angew. Chem. 2014, 126, 4444.
- 2For a review for the synthesis of 3,4-fused indoles, see:
- 2aD. Shan, Y. Jia, Chin. J. Org. Chem. 2013, 33, 1144;
- 2bQ. Liu, Y.-A. Zhang, P. Xu, Y. Jia, J. Org. Chem. 2013, 78, 10885;
- 2cY.-A. Zhang, Q. Liu, C. Wang, Y. Jia, Org. Lett. 2013, 15, 3662;
- 2dQ. Liu, Q. Li, Y. Ma, Y. Jia, Org. Lett. 2013, 15, 4528;
- 2eL. Guo, F. Zhang, W. Hu, L. Li, Y. Jia, Chem. Commun. 2014, 50, 3299, and references therein.
- 3
- 3aD. Shan, Y. Gao, Y. Jia, Angew. Chem. Int. Ed. 2013, 52, 4902; Angew. Chem. 2013, 125, 5002;
- 3bY. Gao, D. Shan, Y. Jia, Tetrahedron 2014, 70, 5136;
- 3cfor a similar work, see: S. P. Breazzano, Y. B. Poudel, D. L. Boger, J. Am. Chem. Soc. 2013, 135, 1600.
- 4For a review, see:
- 4aJ. Marco-Contelles, M. C. Carreiras, C. Rodríguez, M. Villarroya, A. G. García, Chem. Rev. 2006, 106, 116; for racemic syntheses reported after those cited in Ref. [4a], see:
- 4bX.-D. Hu, Y.-Q. Tu, E. Zhang, S. Gao, S. Wang, A. Wang, C.-A. Fan, M. Wang, Org. Lett. 2006, 8, 1823;
- 4cT. Ishikawa, K. Kudo, K. Kuroyabu, S. Uchida, T. Kudoh, S. Saito, J. Org. Chem. 2008, 73, 7498;
- 4dM. G. Banwell, X. Ma, O. P. Karunaratne, A. C. Willis, Aust. J. Chem. 2010, 63, 1437;
- 4eJ. H. Chang, H.-U. Kang, I.-H. Jung, C.-G. Cho, Org. Lett. 2010, 12, 2016;
- 4fY. Feng, Z.-X. Yu, J. Org. Chem. 2015, 80, 1952.
- 5For recent syntheses of lycoramine, see:
- 5aW. P. Malachowski, T. Paul, S. Phounsavath, J. Org. Chem. 2007, 72, 6792;
- 5bC.-A. Fan, Y.-Q. Tu, Z.-L. Song, E. Zhang, L. Shi, M. Wang, B. Wang, S.-Y. Zhang, Org. Lett. 2004, 6, 4691, and references therein.
- 6D. H. R. Barton, G. W. Kirby, J. Chem. Soc. C 1962, 806.
- 7
- 7aK. Tomioka, K. Shimizu, S. Yamada, K. Koga, Heterocycles 1977, 6, 1752;
- 7bK. Shimizu, K. Tomioka, S. Yamada, K. Koga, Heterocycles 1977, 8, 277.
- 8
- 8aS. Kodama, Y. Hamashima, K. Nishide, M. Node, Angew. Chem. Int. Ed. 2004, 43, 2659; Angew. Chem. 2004, 116, 2713;
- 8bM. Node, S. Kodama, Y. Hamashima, T. Katoh, K. Nishide, T. Kajimoto, Chem. Pharm. Bull. 2006, 54, 1662.
- 9
- 9aB. M. Trost, F. D. Toste, J. Am. Chem. Soc. 2000, 122, 11262;
- 9bB. M. Trost, W. Tang, Angew. Chem. Int. Ed. 2002, 41, 2795;
10.1002/1521-3773(20020802)41:15<2795::AID-ANIE2795>3.0.CO;2-2 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 2919;
- 9cB. M. Trost, W. Tang, F. D. Toste, J. Am. Chem. Soc. 2005, 127, 14785.
- 10V. Satcharoen, N. J. McLean, S. C. Kemp, N. P. Camp, R. C. D. Brown, Org. Lett. 2007, 9, 1867.
- 11aH. Tanimoto, T. Kato, N. Chida, Tetrahedron Lett. 2007, 48, 6267 (unnatural galanthamine);
- 11bT. Kato, H. Tanimoto, H. Yamada, N. Chida, Heterocycles 2010, 82, 563.
- 12P. Magnus, N. Sane, B. P. Fauber, V. Lynch, J. Am. Chem. Soc. 2009, 131, 16045.
- 13P. Chen, X. Bao, L.-F. Zhang, M. Ding, X.-J. Han, J. Li, G.-B. Zhang, Y.-Q. Tu, C.-A. Fan, Angew. Chem. Int. Ed. 2011, 50, 8161; Angew. Chem. 2011, 123, 8311.
- 14J.-Q. Chen, J.-H. Xie, D.-H. Bao, S. Liu, Q.-L. Zhou, Org. Lett. 2012, 14, 2714.
- 15Y. Zang, I. Ojima, J. Org. Chem. 2013, 78, 4013.
- 16J. Choi, H. Kim, S. Park, J. Tae, Synlett 2013, 379.
- 17For recent reviews, see:
- 17aI. Marek, Y. Minko, M. Pasco, T. Mejuch, N. Gilboa, H. Chechik, J. P. Das, J. Am. Chem. Soc. 2014, 136, 2682;
- 17bA. Y. Hong, B. M. Stoltz, Eur. J. Org. Chem. 2013, 2745;
- 17cJ. P. Das, I. Marek, Chem. Commun. 2011, 47, 4593;
- 17dC. Hawner, A. Alexakis, Chem. Commun. 2010, 46, 7295;
- 17eS. Jautze, R. Peters, Synthesis 2010, 365; for selective recent examples on the catalytic asymmetric formation of all-carbon quaternary centers in natural product syntheses, see:
- 17fZ. Li, S. Zhang, S. Wu, X. Shen, L. Zou, F. Wang, X. Li, F. Peng, H. Zhang, Z. Shao, Angew. Chem. Int. Ed. 2013, 52, 4117; Angew. Chem. 2013, 125, 4211;
- 17gZ. Xu, Q. Wang, J. Zhu, J. Am. Chem. Soc. 2013, 135, 19127.
- 18
- 18aR. C. Larock, J. Organomet. Chem. 1999, 576, 111;
- 18bR. C. Larock, E. K. Yum, M. J. Doty, K. C. Sham, J. Org. Chem. 1995, 60, 3270.
- 19For the only example of the construction of 3,4-fused furan through intramolecular Larock-type annulation, see:
- 19aA. Kojima, T. Takemoto, M. Sodeoka, M. Shibasaki, J. Org. Chem. 1996, 61, 4876;
- 19bA. Kojima, S. Shimizu, M. Shibasaki, J. Heterocycl. Chem. 1998, 35, 1057.
- 20T. Konno, J. Chae, T. Ishihara, H. Yamanaka, Tetrahedron 2004, 60, 11695.
- 21
- 21aX. Li, Z. Xi, S. Luo, J.-P. Cheng, Adv. Synth. Catal. 2010, 352, 1097;
- 21bX. Li, S. Hu, Z. Xi, L. Zhang, S. Luo, J.-P. Cheng, J. Org. Chem. 2010, 75, 8697;
- 21cF. Pesciaioli, X. Tian, G. Bencivenni, G. Bartoli, P. Melchiorre, Synlett 2010, 1704.
- 22
- 22aX. H. Liu, L. L. Lin, X. M. Feng, Acc. Chem. Res. 2011, 44, 574;
- 22bX. H. Liu, L. L. Lin, X. M. Feng, Org. Chem. Front. 2014, 1, 298.
- 23
- 23aG. A. Kraus, K. A. Frazier, B. D. Roth, M. J. Taschner, K. Neuenschwander, J. Org. Chem. 1981, 46, 2417;
- 23bG. A. Kraus, J. Shi, D. Reynolds, J. Org. Chem. 1990, 55, 1105.
- 24H. T. Clarke, H. B. Gillespie, S. Z. Weisshaus, J. Am. Chem. Soc. 1933, 55, 4571.