The σ-Aromatic Clusters [Zn3]+ and [Zn2Cu]: Embryonic Brass†
This work was funded by the Deutsche Forschungsgemeinschaft (individual grant Fi-502/23-2) and by RESOLV (EXC 1069). The dissertation project of K.F. was supported by the German Chemical Industry Fund (https://vci.de/fonds) and the Ruhr University Research School (http://www.research-school.rub.de/). H.B. is grateful for a scholarship of the German Chemical Industry Fund.
Graphical Abstract
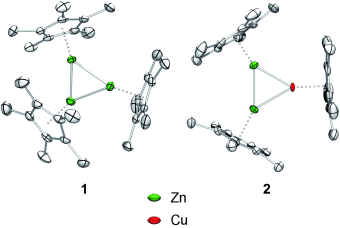
Bold as brass: The bonding situations of the Cp* ligand-protected clusters [Zn3]+ (1) and [Zn2Cu] (2) were investigated by quantum chemical calculations revealing a high degree of σ-aromaticity similar to the trihydrogen ion [H3]+. The new species serve as molecular building units of CunZnm nanobrass clusters as indicated by liquid-injection field desorption ionization (LIFDI) mass spectrometry.
Abstract
The triangular clusters [Zn3Cp*3]+ and [Zn2CuCp*3] were obtained by addition of the in situ generated, electrophilic, and isolobal species [ZnCp*]+ and [CuCp*] to Carmona’s compound, [Cp*ZnZnCp*], without splitting the ZnZn bond. The choice of non-coordinating fluoroaromatic solvents was crucial. The bonding situations of the all-hydrocarbon-ligand-protected clusters were investigated by quantum chemical calculations revealing a high degree of σ-aromaticity similar to the triatomic hydrogen ion [H3]+. The new species serve as molecular building units of CunZnm nanobrass clusters as indicated by LIFDI mass spectrometry.