Multidimensional De Novo Design Reveals 5-HT2B Receptor-Selective Ligands†
Dr. Tiago Rodrigues
Department of Chemistry and Applied Biosciences, Swiss Federal Institute of Technology (ETH), Vladimir-Prelog-Weg 4, 8093 Zurich (Switzerland)
These authors contributed equally to this work.
Search for more papers by this authorNadine Hauser
Department of Chemistry and Applied Biosciences, Swiss Federal Institute of Technology (ETH), Vladimir-Prelog-Weg 4, 8093 Zurich (Switzerland)
These authors contributed equally to this work.
Search for more papers by this authorDaniel Reker
Department of Chemistry and Applied Biosciences, Swiss Federal Institute of Technology (ETH), Vladimir-Prelog-Weg 4, 8093 Zurich (Switzerland)
Search for more papers by this authorDr. Michael Reutlinger
Department of Chemistry and Applied Biosciences, Swiss Federal Institute of Technology (ETH), Vladimir-Prelog-Weg 4, 8093 Zurich (Switzerland)
Search for more papers by this authorTiffany Wunderlin
Novartis Institutes for BioMedical Research (NIBR), Novartis AG, 4056 Basel (Switzerland)
Search for more papers by this authorDr. Jacques Hamon
Novartis Institutes for BioMedical Research (NIBR), Novartis AG, 4056 Basel (Switzerland)
Search for more papers by this authorDr. Guido Koch
Novartis Institutes for BioMedical Research (NIBR), Novartis AG, 4056 Basel (Switzerland)
Search for more papers by this authorCorresponding Author
Prof. Dr. Gisbert Schneider
Department of Chemistry and Applied Biosciences, Swiss Federal Institute of Technology (ETH), Vladimir-Prelog-Weg 4, 8093 Zurich (Switzerland)
Department of Chemistry and Applied Biosciences, Swiss Federal Institute of Technology (ETH), Vladimir-Prelog-Weg 4, 8093 Zurich (Switzerland)Search for more papers by this authorDr. Tiago Rodrigues
Department of Chemistry and Applied Biosciences, Swiss Federal Institute of Technology (ETH), Vladimir-Prelog-Weg 4, 8093 Zurich (Switzerland)
These authors contributed equally to this work.
Search for more papers by this authorNadine Hauser
Department of Chemistry and Applied Biosciences, Swiss Federal Institute of Technology (ETH), Vladimir-Prelog-Weg 4, 8093 Zurich (Switzerland)
These authors contributed equally to this work.
Search for more papers by this authorDaniel Reker
Department of Chemistry and Applied Biosciences, Swiss Federal Institute of Technology (ETH), Vladimir-Prelog-Weg 4, 8093 Zurich (Switzerland)
Search for more papers by this authorDr. Michael Reutlinger
Department of Chemistry and Applied Biosciences, Swiss Federal Institute of Technology (ETH), Vladimir-Prelog-Weg 4, 8093 Zurich (Switzerland)
Search for more papers by this authorTiffany Wunderlin
Novartis Institutes for BioMedical Research (NIBR), Novartis AG, 4056 Basel (Switzerland)
Search for more papers by this authorDr. Jacques Hamon
Novartis Institutes for BioMedical Research (NIBR), Novartis AG, 4056 Basel (Switzerland)
Search for more papers by this authorDr. Guido Koch
Novartis Institutes for BioMedical Research (NIBR), Novartis AG, 4056 Basel (Switzerland)
Search for more papers by this authorCorresponding Author
Prof. Dr. Gisbert Schneider
Department of Chemistry and Applied Biosciences, Swiss Federal Institute of Technology (ETH), Vladimir-Prelog-Weg 4, 8093 Zurich (Switzerland)
Department of Chemistry and Applied Biosciences, Swiss Federal Institute of Technology (ETH), Vladimir-Prelog-Weg 4, 8093 Zurich (Switzerland)Search for more papers by this authorN.H. and T.R. performed organic syntheses. D.R. and M.R. contributed computational tools and performed in silico experiments together with N.H. and T.R. T.W., J.H., and G.K. contributed binding assays. All authors analyzed and discussed data. T.R. and G.S. designed the study and wrote the manuscript with feedback from the remaining authors.
Graphical Abstract
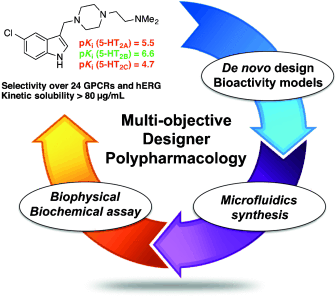
Multi-objective design: Multidimensional de novo design generated innovative and nanomolar-potent 5-HT2B-selective antagonists. Computational bioaffinity prediction for full target panels and microfluidics-assisted synthesis facilitated their discovery. Our results suggest that such integrated discovery platforms will find further applicability in swift prototyping of drug candidates.
Abstract
We report a multi-objective de novo design study driven by synthetic tractability and aimed at the prioritization of computer-generated 5-HT2B receptor ligands with accurately predicted target-binding affinities. Relying on quantitative bioactivity models we designed and synthesized structurally novel, selective, nanomolar, and ligand-efficient 5-HT2B modulators with sustained cell-based effects. Our results suggest that seamless amalgamation of computational activity prediction and molecular design with microfluidics-assisted synthesis enables the swift generation of small molecules with the desired polypharmacology.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201410201_sm_miscellaneous_information.pdf3.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. Rodrigues, G. Schneider, Synlett 2014, 25, 170–178;
- 1bY. L. Bennani, Drug Discovery Today 2011, 16, 779–792.
- 2
- 2aA. L. Hopkins, Nat. Chem. Biol. 2008, 4, 682–690;
- 2bJ. Besnard, G. F. Ruda, V. Setola, K. Abecassis, R. M. Rodriguiz, X. P. Huang, S. Norval, M. F. Sassano, A. I. Shin, L. A. Webster, F. R. Simeons, L. Stojanovski, A. Prat, N. G. Seidah, D. B. Constam, G. R. Bickerton, K. D. Read, W. C. Wetsel, I. H. Gilbert, B. L. Roth, A. L. Hopkins, Nature 2012, 492, 215–220;
- 2cH. Lin, M. F. Sassano, B. L. Roth, B. K. Shoichet, Nat. Methods 2013, 10, 140–146;
- 2dE. Lounkine, M. J. Keiser, S. Whitebread, D. Mikhailov, J. Hamon, J. L. Jenkins, P. Lavan, E. Weber, A. K. Doak, S. Cote, B. K. Shoichet, L. Urban, Nature 2012, 486, 361–367;
- 2eM. J. Keiser, B. L. Roth, B. N. Armbruster, P. Ernsberger, J. J. Irwin, B. K. Shoichet, Nat. Biotechnol. 2007, 25, 197–206;
- 2fD. Reker, T. Rodrigues, P. Schneider, G. Schneider, Proc. Natl. Acad. Sci. USA 2014, 111, 4067–4072.
- 3
- 3aT. Rodrigues, T. Kudoh, F. Roudnicky, Y. F. Lim, Y.-C. Lin, C. P. Koch, M. Seno, M. Detmar, G. Schneider, Angew. Chem. Int. Ed. 2013, 52, 10006–10009; Angew. Chem. 2013, 125, 10190–10193;
- 3bB. Spänkuch, S. Keppner, L. Lange, T. Rodrigues, H. Zettl, C. P. Koch, M. Reutlinger, M. Hartenfeller, P. Schneider, G. Schneider, Angew. Chem. Int. Ed. 2013, 52, 4676–4681; Angew. Chem. 2013, 125, 4774–4779;
- 3cT. Rodrigues, F. Roudnicky, C. P. Koch, T. Kudoh, D. Reker, M. Detmar, G. Schneider, Chem. Sci. 2013, 4, 1229–1233.
- 4
- 4aT. Rodrigues, P. Schneider, G. Schneider, Angew. Chem. Int. Ed. 2014, 53, 5750–5758; Angew. Chem. 2014, 126, 5858–5866;
- 4bK. S. Elvira, X. Casadevall i Solvas, R. C. Wootton, A. J. deMello, Nat. Chem. 2013, 5, 905–915.
- 5
- 5aM. Berger, J. A. Gray, B. L. Roth, Annu. Rev. Med. 2009, 60, 355–366;
- 5bN. Moss, Y. Choi, D. Cogan, A. Flegg, A. Kahrs, P. Loke, O. Meyn, R. Nagaraja, S. Napier, A. Parker, J. T. Peterson, P. Ramsden, C. Sarko, D. Skow, J. Tomlinson, H. Tye, M. Whitaker, Bioorg. Med. Chem. Lett. 2009, 19, 2206–2210;
- 5cY. J. Kwon, S. Saubern, J. M. Macdonald, X. P. Huang, V. Setola, B. L. Roth, Bioorg. Med. Chem. Lett. 2010, 20, 5488–5490;
- 5d Encyclopedia of Molecular Pharmacology, 2nd ed. (Eds.: ), Springer, Heidelberg, 2008, p. 1125.
- 6J. Hamon, S. Whitebread, V. Techer-Etienne, H. Le Coq, K. Azzaoui, L. Urban, Future Med. Chem. 2009, 1, 645–665.
- 7M. Reutlinger, T. Rodrigues, P. Schneider, G. Schneider, Angew. Chem. Int. Ed. 2014, 53, 4244–4248; Angew. Chem. 2014, 126, 4330–4334.
- 8M. Reutlinger, T. Rodrigues, P. Schneider, G. Schneider, Angew. Chem. Int. Ed. 2014, 53, 582–585; Angew. Chem. 2014, 126, 593–596.
- 9M. Reutlinger, C. P. Koch, D. Reker, N. Todoroff, P. Schneider, T. Rodrigues, G. Schneider, Mol. Inf. 2013, 32, 133–138.
- 10D. Rogers, M. Hahn, J. Chem. Inf. Model. 2010, 50, 742–754.
- 11J. E. Hochlowski, P. A. Searle, N. P. Tu, J. Y. Pan, S. G. Spanton, S. W. Djuric, J. Flow Chem. 2011, 2, 56–61.
- 12M. F. Sassano, A. K. Doak, B. L. Roth, B. K. Shoichet, J. Med. Chem. 2013, 56, 2406–2414.
- 13J. Bowes, A. J. Brown, J. Hamon, W. Jarolimek, A. Sridhar, G. Waldron, S. Whitebread, Nat. Rev. Drug Discovery 2012, 11, 909–922.
- 14A. Gaulton, L. J. Bellis, A. P. Bento, J. Chambers, M. Davies, A. Hersey, Y. Light, S. McGlinchey, D. Michalovich, B. Al-Lazikani, J. P. Overington, Nucleic Acids Res. 2012, 40, D 1100–D1107.
- 15J. H. Lange, J. H. Reinders, J. T. Tolboom, J. C. Glennon, H. K. Coolen, C. G. Kruse, J. Med. Chem. 2007, 50, 5103–5108.
- 16I. A. Moussa, S. D. Banister, C. Beinat, N. Giboureau, A. J. Reynolds, M. Kassiou, J. Med. Chem. 2010, 53, 6228–6239.
- 17O. M. Becker, D. S. Dhanoa, Y. Marantz, D. Chen, S. Shacham, S. Cheruku, A. Heifetz, P. Mohanty, M. Fichman, A. Sharadendu, R. Nudelman, M. Kauffman, S. Noiman, J. Med. Chem. 2006, 49, 3116–3135.
- 18M. Reutlinger, W. Guba, R. E. Martin, A. I. Alanine, T. Hoffmann, A. Klenner, J. A. Hiss, P. Schneider, G. Schneider, Angew. Chem. Int. Ed. 2011, 50, 11633–11636; Angew. Chem. 2011, 123, 11837–11840.
- 19M. Hartenfeller, H. Zettl, M. Walter, M. Rupp, F. Reisen, E. Proschak, S. Weggen, H. Stark, G. Schneider, PLoS Comput. Biol. 2012, 8, e 1002380.
- 20J. B. Baell, G. A. Holloway, J. Med. Chem. 2010, 53, 2719–2740.
- 21D. Lagorce, O. Sperandio, H. Galons, M. A. Miteva, B. O. Villoutreix, BMC Bioinf. 2008, 9, 396.
- 22S. P. Alexander, A. Mathie, J. A. Peters, Br. J. Pharmacol. 2009, 158, S 1–254.
- 23J. A. May, A. P. Dantanarayana, P. W. Zinke, M. A. McLaughlin, N. A. Sharif, J. Med. Chem. 2006, 49, 318–328.
- 24J. D. Urban, G. A. Vargas, M. von Zastrow, R. B. Mailman, Neuropsychopharmacology 2007, 32, 67–77.
- 25S. P. Alexander, A. Mathie, J. A. Peters, Br. J. Pharmacol. 2011, 164, S 1–324.
- 26D. W. Bonhaus, L. A. Flippin, R. J. Greenhouse, S. Jaime, C. Rocha, M. Dawson, K. Van Natta, L. K. Chang, T. Pulido-Rios, A. Webber, E. Leung, R. M. Eglen, G. R. Martin, Br. J. Pharmacol. 1999, 127, 1075–1082.
- 27S. L. Porvasnik, S. Germain, J. Embury, K. S. Gannon, V. Jacques, J. Murray, B. J. Byrne, S. Shacham, R. Al-Mousily, J. Pharmacol. Exp. Ther. 2010, 334, 364–372.
- 28W. E. Evans, J. A. Johnson, Annu. Rev. Genomics Hum. Genet. 2001, 2, 9–39.