Divergent Mechanistic Routes for the Formation of gem-Dimethyl Groups in the Biosynthesis of Complex Polyketides†
The authors would like to thank Isu Yoon for assistance with protein purification and Satoshi Yuzawa for helpful discussions. This work was supported by the Joint BioEnergy Institute, which is funded by the Office of Science, Office of Biological and Environmental Research of the U.S. Department of Energy (Contract No. DE-AC02-05CH11231), by the National Science Foundation (award No. EEC-0540879 to the Synthetic Biology Research Center), by the Department of Energy, ARPA-E Electrofuels Program (Contract No. DE-0000206-1577), and by the National Science Foundation Graduate Research Fellowship Program (Grant No. DGE 1106400, to SP).
Graphical Abstract
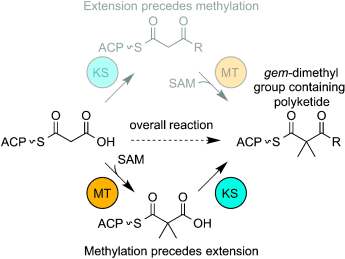
Order of events: In order to elucidate the mechanism of gem-dimethyl group formation in polyketides, the gem-dimethyl group producing polyketide synthase (PKS) modules of yersiniabactin and epothilone were characterized using mass spectrometry. The study demonstrated, contrary to the canonical understanding of reaction order in PKSs, that methylation can precede condensation in PKS modules that produce gem-dimethyl groups.
Abstract
The gem-dimethyl groups in polyketide-derived natural products add steric bulk and, accordingly, lend increased stability to medicinal compounds, however, our ability to rationally incorporate this functional group in modified natural products is limited. In order to characterize the mechanism of gem-dimethyl group formation, with a goal toward engineering of novel compounds containing this moiety, the gem-dimethyl group producing polyketide synthase (PKS) modules of yersiniabactin and epothilone were characterized using mass spectrometry. The work demonstrated, contrary to the canonical understanding of reaction order in PKSs, that methylation can precede condensation in gem-dimethyl group producing PKS modules. Experiments showed that both PKSs are able to use dimethylmalonyl acyl carrier protein (ACP) as an extender unit. Interestingly, for epothilone module 8, use of dimethylmalonyl-ACP appeared to be the sole route to form a gem-dimethylated product, while the yersiniabactin PKS could methylate before or after ketosynthase condensation.