A Cationic Zinc Hydride Cluster Stabilized by an N-Heterocyclic Carbene: Synthesis, Reactivity, and Hydrosilylation Catalysis†
Dr. Arnab Rit
Institute of Inorganic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Search for more papers by this authorDr. Alessandro Zanardi
Institute of Inorganic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Search for more papers by this authorDr. Thomas P. Spaniol
Institute of Inorganic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Laurent Maron
Université de Toulouse et CNRS, INSA, UPS, CNRS; UMR 5215 LPCNO, 135 avenue de Rangueli, F-31077 Toulouse (France)
Laurent Maron, Université de Toulouse et CNRS, INSA, UPS, CNRS; UMR 5215 LPCNO, 135 avenue de Rangueli, F-31077 Toulouse (France)
Jun Okuda, Institute of Inorganic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jun Okuda
Institute of Inorganic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Laurent Maron, Université de Toulouse et CNRS, INSA, UPS, CNRS; UMR 5215 LPCNO, 135 avenue de Rangueli, F-31077 Toulouse (France)
Jun Okuda, Institute of Inorganic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Search for more papers by this authorDr. Arnab Rit
Institute of Inorganic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Search for more papers by this authorDr. Alessandro Zanardi
Institute of Inorganic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Search for more papers by this authorDr. Thomas P. Spaniol
Institute of Inorganic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Laurent Maron
Université de Toulouse et CNRS, INSA, UPS, CNRS; UMR 5215 LPCNO, 135 avenue de Rangueli, F-31077 Toulouse (France)
Laurent Maron, Université de Toulouse et CNRS, INSA, UPS, CNRS; UMR 5215 LPCNO, 135 avenue de Rangueli, F-31077 Toulouse (France)
Jun Okuda, Institute of Inorganic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jun Okuda
Institute of Inorganic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Laurent Maron, Université de Toulouse et CNRS, INSA, UPS, CNRS; UMR 5215 LPCNO, 135 avenue de Rangueli, F-31077 Toulouse (France)
Jun Okuda, Institute of Inorganic Chemistry, RWTH Aachen University, Landoltweg 1, 52056 Aachen (Germany)
Search for more papers by this authorWe thank the Deutsche Forschungsgemeinschaft for financial support through the International Research Training Group “Selectivity in Chemo- and Biocatalysis” (GRK 1628). L.M. is a fellow of the Alexander von Humboldt Foundation. We thank Toni Gossen for assistance with the NMR spectroscopy.
Graphical Abstract
Abstract
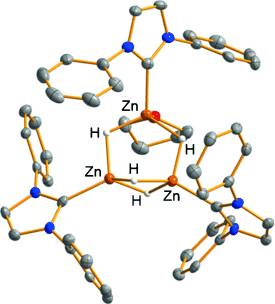
The trinuclear cationic zinc hydride cluster [(IMes)3Zn3H4(THF)](BPh4)2 (1) was obtained either by protonation of the neutral zinc dihydride [(IMes)ZnH2]2 with a Brønsted acid or by addition of the putative zinc dication [(IMes)Zn(THF)]2+. A triply bridged thiophenolato complex 2 was formed upon oxidation of 1 with PhSSPh. Protonolysis of 1 by methanol or water gave the corresponding trinuclear dicationic derivatives. At ambient temperature, 1 catalyzed the hydrosilylation of aldehydes, ketones, and nitriles. Carbon dioxide was also hydrosilylated under forcing conditions when using (EtO)3SiH, giving silylformate as the main product.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201408346_sm_miscellaneous_information.pdf1,011.4 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. E. Finholt, A. C. Bond, Jr., H. I. Schlesinger, J. Am. Chem. Soc. 1947, 69, 1199–1203;
- 1bJ. J. Watkins, E. C. Ashby, Inorg. Chem. 1974, 13, 2350–2354;
- 1cE. C. Ashby, J. J. Watkins, D. Greig, D. F. Shriver, Inorg. Synth. 1977, 17, 6–8;
- 1dA. J. De Koning, J. Boersma, G. J. M. Van Der Kerk, J. Organomet. Chem. 1980, 186, 159–172;
- 1eE. C. Ashby, A. B. Goel, J. Organomet. Chem. 1981, 204, 139–145;
- 1fFor gas phase structure, see: A. Shayesteh, D. R. T. Appadoo, I. E. Gordon, P. F. Bernath, J. Am. Chem. Soc. 2004, 126, 14356–14357;
- 1gFor noble gas matrix isolation study, see: T. M. Greene, W. Brown, L. Andrews, A. J. Downs, G. V. Chertihin, N. Rueneberg, P. Pyykkö, J. Phys. Chem. 1995, 99, 7925–7934.
- 2
- 2aY. Gao, H. Urabe, F. Sato, J. Org. Chem. 1994, 59, 5521–5523;
- 2bY. Gao, K. Harada, T. Hata, H. Urabe, F. Sato, J. Org. Chem. 1995, 60, 290–291.
- 3A. Rit, T. P. Spaniol, L. Maron, J. Okuda, Angew. Chem. Int. Ed. 2013, 52, 4664–4667; Angew. Chem. 2013, 125, 4762–4765.
- 4For selected references, see:
- 4aA. Looney, R. Han, I. B. Gorrell, M. Cornebise, K. Yoon, G. Parkin, A. L. Rheingold, Organometallics 1995, 14, 274–278;
- 4bH. Hao, C. Cui, H. W. Roesky, G. Bai, H.-G. Schmidt, M. Noltemeyer, Chem. Commun. 2001, 1118–1119;
- 4cM. P. Coles, S. M. El-Hamruni, J. D. Smith, P. B. Hitchcock, Angew. Chem. Int. Ed. 2008, 47, 10147–10150; Angew. Chem. 2008, 120, 10301–10304;
- 4dJ. Spielmann, D. Piesik, B. Wittkamp, G. Jansen, S. Harder, Chem. Commun. 2009, 3455–3456;
- 4eB. Gutschank, S. Schulz, D. Bläser, R. Boese, C. Wölper, Organometallics 2010, 29, 6133–6136;
- 4fD. Mukherjee, A. Ellern, A. D. Sadow, J. Am. Chem. Soc. 2010, 132, 7582–7583;
- 4gW. Sattler, G. Parkin, J. Am. Chem. Soc. 2011, 133, 9708–9711;
- 4hN. J. Brown, J. E. Harris, X. Yin, I. Silverwood, A. J. P. White, S. G. Kazarian, K. Hellgardt, M. S. P. Shaffer, C. K. Williams, Organometallics 2014, 33, 1112–1119;
- 4iP. Jochmann, D. W. Stephan, Chem. Commun. 2014, 50, 8395–8397.
- 5
- 5aG. J. Kubas, D. F. Shriver, J. Am. Chem. Soc. 1970, 92, 1949–1954;
- 5bD. F. Shriver, G. J. Kubas, J. A. Marshall, J. Am. Chem. Soc. 1971, 93, 5076–5079;
- 5cE. C. Ashby, R. G. Beach, Inorg. Chem. 1971, 10, 2486–2488;
- 5dE. C. Ashby, J. J. Watkins, Inorg. Chem. 1973, 12, 2493–2503;
- 5eA. Lennartson, M. Håkansson, S. Jagner, Angew. Chem. Int. Ed. 2007, 46, 6678–6680; Angew. Chem. 2007, 119, 6798–6800;
- 5fP. A. Lummis, M. R. Momeni, M. W. Lui, R. McDonald, M. J. Ferguson, M. Miskolzic, A. Brown, E. Rivard, Angew. Chem. Int. Ed. 2014, 53, 9347–9351; Angew. Chem. 2014, 126, 9501–9505.
- 6For reviews on N-heterocyclic carbenes, see:
- 6aF. E. Hahn, M. C. Jahnke, Angew. Chem. Int. Ed. 2008, 47, 3122–3172; Angew. Chem. 2008, 120, 3166–3216;
- 6bM. Melaimi, M. Soleilhavoup, G. Bertrand, Angew. Chem. Int. Ed. 2010, 49, 8810–8849; Angew. Chem. 2010, 122, 8992–9032.
- 7D. Wang, K. Wurst, M. R. Buchmeiser, J. Organomet. Chem. 2004, 689, 2123–2130.
- 8
- 8aZ. Zhu, R. J. Wright, M. M. Olmstead, E. Rivard, M. Brynda, P. P. Power, Angew. Chem. Int. Ed. 2006, 45, 5807–5810; Angew. Chem. 2006, 118, 5939–5942;
- 8bZ. Zhu, M. Brynda, R. J. Wright, R. C. Fischer, W. A. Merrill, E. Rivard, R. Wolf, J. C. Fettinger, M. A. Olmstead, P. P. Power, J. Am. Chem. Soc. 2007, 129, 10847–10857.
- 9
- 9aF. A. Cotton, G. Schmid, Polyhedron 1996, 15, 4053–4059;
- 9bD. J. Darensbourg, M. S. Zimmer, P. Rainey, D. L. Larkins, Inorg. Chem. 2000, 39, 1578–1585.
- 10W. J. Evans, S. A. Kozimer, J. W. Zeller, Chem. Commun. 2005, 4681–4683.
- 11
- 11aD. L. Reger, A. E. Pascui, P. J. Pellechia, M. D. Smith, Inorg. Chem. 2013, 52, 11638–11649;
- 11bG. Anantharaman, K. Elango, Organometallics 2007, 26, 1089–1092.
- 12
- 12aW. Sattler, G. Parkin, J. Am. Chem. Soc. 2012, 134, 17462–17465;
- 12bD. Mukherjee, R. R. Thompson, A. Ellern, A. D. Sadow, ACS Catal. 2011, 1, 698–702;
- 12cP. Jochmann, D. W. Stephan, Angew. Chem. Int. Ed. 2013, 52, 9831–9835; Angew. Chem. 2013, 125, 10014–10018;
- 12dC. Boone, I. Korobkov, G. I. Nikonov, ACS Catal. 2013, 3, 2336–2340;
- 12eA. Rit, T. P. Spaniol, J. Okuda, Chem. Asian J. 2014, 9, 612–619.
- 13 The Chemistry of Organic Silicon Compounds, Vol. 3 (Eds.: ), Wiley, New York, 2001.
- 14For reviews on hydrosilylation, see:
- 14a Hydrosilylation: A Comprehensive Review on Recent Advances (Ed.: ), Springer, Heidelberg, 2009;
- 14bO. Riant, N. Mostefaï, J. Courmarcel, Synthesis 2004, 2943–2958.
- 15Hydrosilylation of aldehydes with (EtO)3SiH gave a mixture of silyl ethers (R1CH2O)xSi(OEt)4−x (x=0–3) by redistribution of alkoxide ligands, which has also been observed previously;[12d, 16a] this is presumably catalyzed by a metal hydride species.
- 16Selected references:
- 16aE. Peterson, A. Y. Khalimon, R. Simionescu, L. G. Kuzmina, J. A. K. Howard, G. I. Nikonov, J. Am. Chem. Soc. 2009, 131, 908–909;
- 16bS. Chakraborty, J. A. Krause, H. Guan, Organometallics 2009, 28, 582–586.
- 17
- 17aH. Kaur, F. K. Zinn, E. D. Stevens, S. P. Nolan, Organometallics 2004, 23, 1157–1160;
- 17bS. Díez-González, N. M. Scott, S. P. Nolan, Organometallics 2006, 25, 2355–2358;
- 17cS. Díez-González, E. D. Stevens, N. M. Scott, J. L. Petersen, S. P. Nolan, Chem. Eur. J. 2008, 14, 158–168.
- 18
- 18aI. Ojima, T. Kogure, M. Kumagai, S. Horiuchi, T. Sato, J. Organomet. Chem. 1976, 122, 83–97;
- 18bG. Du, P. E. Fanwick, M. M. Abu-Omar, J. Am. Chem. Soc. 2004, 126, 8072–8073;
- 18cT. Steinke, C. Gemel, M. Cokoja, M. Winter, R. A. Fischer, Angew. Chem. Int. Ed. 2004, 43, 2299–2302; Angew. Chem. 2004, 116, 2349–2352.
- 19CCDC 1014080 (1), 1014081 (2), and 1014082 (3 b) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.